Monocyte Chemotactic Protein-1 as a Potential Biomarker for Early Anti-Thrombotic Therapy after Ischemic Stroke
Abstract
:1. Introduction
2. Results
2.1. Study Population
2.2. MCP-1 Levels after Differential Antithrombotic Treatment
2.3. Outcome at 90 Days Dependent on Stroke Severity but not on MCP-1
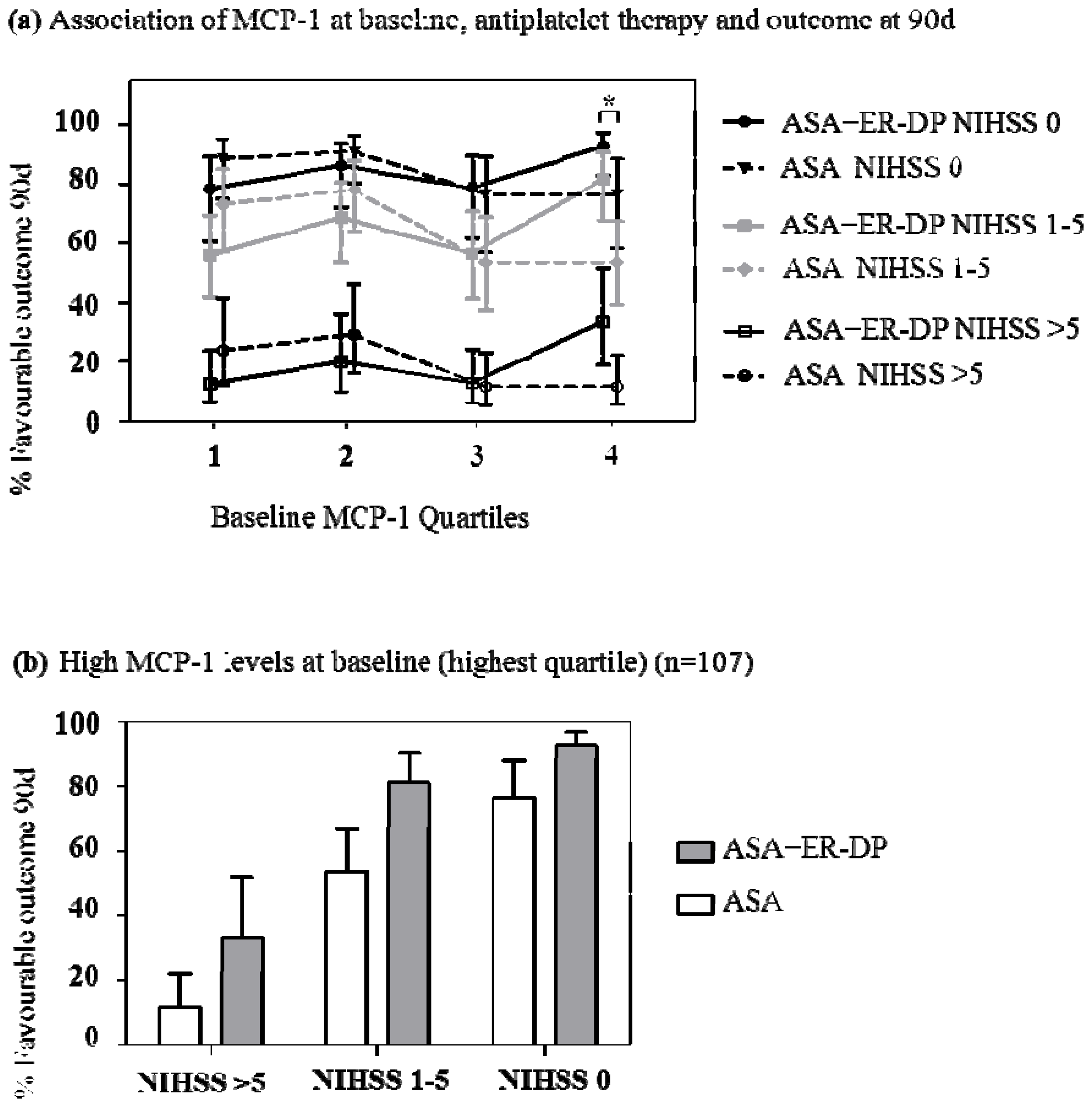
2.4. Association of MCP-1 Baseline Levels, Anti-Thrombotic Therapy and Outcome at 90 Days
3. Discussion
4. Experimental Section
4.1. Patients and Clinical Assessment
4.2. Protocol Approval, Registration and Patient Consents
4.3. Procedures
4.4. Measurement of MCP-1
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
- Conflict of InterestFor H.W., there is no conflict of interest. R.D. has received consulting fees, honoraria, and support for travel to meetings for the study, fees for participation in review activities, payment for development of educational presentations, and travel expenses from Boehringer Ingelheim. H.S. is and W.G.E. has been employees of Boehringer Ingelheim Pharma GmbH and Co. KG. A.S. has received consulting fees, honoraria, support for travel to meetings for the study, fees for participation in review activities, and board membership fees from Boehringer Ingelheim, and honoraria from Sanofi–Aventis. R.L. has received consulting fees, and honoraria from Boehringer Ingelheim. K.W. has received consulting fees and support for travel to meetings for the study from Boehringer Ingelheim. The study was sponsored by Boehringer Ingelheim. The sponsor was involved in the study design, data collection, data analysis, and interpretation of the data. The sponsor was not involved in the decision to submit the paper for publication. All authors had complete access to the trial data and had responsibility for the final statistical analysis and interpretation of the results. All authors vouch for the accuracy and completeness of the data and analyses. The corresponding author was responsible for the decision to submit the paper for publication.
References
- Mergenthaler, P.; Dirnagl, U.; Meisel, A. Pathophysiology of stroke: Lessons from animal models. Metab. Brain Dis 2004, 19, 151–167. [Google Scholar]
- Rodríguez-Yáñez, M.; Castillo, J. Role of inflammatory markers in brain ischemia. Curr. Opin. Neurol 2008, 21, 353–357. [Google Scholar]
- Worthmann, H.; Tryc, A.B.; Goldbecker, A.; Ma, Y.T.; Tountopoulou, A.; Hahn, A.; Dengler, R.; Lichtinghagen, R.; Weissenborn, K. The temporal profile of inflammatory markers and mediators in blood after acute ischemic stroke differs depending on stroke outcome. Cerebrovasc. Dis 2010, 30, 85–92. [Google Scholar]
- Welsh, P.; Barber, M.; Langhorne, P.; Rumley, A.; Lowe, G.D.; Stott, D.J. Associations of inflammatory biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc. Dis 2009, 27, 247–253. [Google Scholar]
- Foerch, C.; Montaner, J.; Furie, K.L.; Ning, M.M.; Lo, E.H. Invited article: searching for oracles? Blood biomarkers in acute stroke. Neurology 2009, 73, 393–399. [Google Scholar]
- Chen, Y.; Hallenbeck, J.M.; Ruetzler, C.; Bol, D.; Thomas, K.; Berman, N.E.; Vogel, S.N. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J. Cereb. Blood Flow Metab 2003, 23, 748–755. [Google Scholar]
- Justicia, C.; Panes, J.; Sole, S.; Cervera, A.; Deulofeu, R.; Chamorro, A.; Planas, A.M. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J. Cereb. Blood Flow Metab 2003, 23, 1430–1440. [Google Scholar]
- Weyrich, A.S.; Denis, M.M.; Kuhlmann-Eyre, J.R.; Spencer, E.D.; Dixon, D.A.; Marathe, G.K.; McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M. Dipyridamole selectively inhibits inflammatory gene expression in platelet-monocyte aggregates. Circulation 2005, 111, 633–642. [Google Scholar]
- Suzuki, S.; Sugai, K.; Sato, H.; Sakatume, M.; Arakawa, M. Inhibition of active oxygen generation by dipyridamole in human polymorphonuclear leukocytes. Eur. J. Pharmacol 1992, 227, 395–401. [Google Scholar]
- Chakrabarti, S.; Vitseva, O.; Iyu, D.; Varghese, S.; Freedman, J.E. The effect of dipyridamole on vascular cell-derived reactive oxygen species. J. Pharmacol. Exp. Ther 2005, 315, 494–500. [Google Scholar]
- Chakrabarti, S.; Freedman, J.E. Dipyridamole, cerebrovascular disease, and the vasculature. Vasc. Pharmacol 2008, 48, 143–149. [Google Scholar]
- Al-Bahrani, A.; Taha, S.; Shaath, H.; Bakhiet, M. TNF-alpha and IL-8 in acute stroke and the modulation of these cytokines by antiplatelet agents. Curr. Neurovasc. Res 2007, 4, 31–37. [Google Scholar]
- Hallevi, H.; Hazan-Halevy, I.; Paran, E. Modification of neutrophil adhesion to human endothelial cell line in acute ischemic stroke by dipyridamole and candesartan. Eur. J. Neurol 2007, 14, 1002–1007. [Google Scholar]
- Offner, H.; Subramanian, S.; Parker, S.M.; Afentoulis, M.E.; Vandenbark, A.A.; Hurn, P.D. Experimental stroke induces massive, rapid activation of the peripheral immune system. J. Cereb. Blood Flow Metab 2006, 26, 654–665. [Google Scholar]
- Conductier, G.; Blondeau, N.; Guyon, A.; Nahon, J.L.; Rovère, C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J. Neuroimmunol 2010, 224, 93–100. [Google Scholar]
- Hughes, P.M.; Allegrini, P.R.; Rudin, M.; Perry, V.H.; Mir, A.K.; Wiessner, C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J. Cereb. Blood Flow Metab 2002, 22, 308–317. [Google Scholar]
- Strecker, J.K.; Minnerup, J.; Gess, B.; Ringelstein, E.B.; Schäbitz, W.R.; Schilling, M. Monocyte chemoattractant protein-1-deficiency impairs the expression of IL-6, IL-1β and G-CSF after transient focal ischemia in mice. PLoS One 2011, 6, e25863. [Google Scholar]
- Dimitrijevic, O.B.; Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 2007, 38, 1345–1353. [Google Scholar]
- Kim, H.H.; Liao, J.K. Translational therapeutics of dipyridamole. Arterioscler. Thromb. Vasc. Biol 2008, 28, s39–s42. [Google Scholar]
- Guo, S.; Stins, M.; Ning, M.; Lo, E.H. Amelioration of inflammation and cytotoxicity by dipyridamole in brain endothelial cells. Cerebrovasc. Dis 2010, 30, 290–296. [Google Scholar]
- García-Bonilla, L.; Sosti, V.; Campos, M.; Penalba, A.; Boada, C.; Sumalla, M.; Hernández-Guillamon, M.; Rosell, A.; Montaner, J. Effects of acute post-treatment with dipyridamole in a rat model of focal cerebral ischemia. Brain Res 2011, 1373, 211–220. [Google Scholar]
- Dengler, R.; Diener, H.C.; Schwartz, A.; Grond, M.; Schumacher, H.; Machnig, T.; Eschenfelder, C.C.; Leonard, J.; Weissenborn, K.; Kastrup, A.; et al. EARLY Investigators. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): A randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010, 9, 159–166. [Google Scholar]
- Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Proceeding of 48th World Medical Association General Assembly, Somerset West, South Africa, 22–26 October, 1996; Available online: http://www.wma.net/en/30publications/10policies/b3/index.html accessed on 1 May 2012.
- Guideline for Good Clinical Practice E6(R1). International Conference on Harmonisation Harmonised Tripartite Guideline. 1996. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf accessed on 1 May 2012.
| Total (n = 425) | Early ASA + ER-DP (n = 220) | Early ASA (n = 205) | |
|---|---|---|---|
| Age (years) ≥ 65 | 68 (27–95) | 67.0 (27–95) | 69.0 (37–88) |
| 272 (64%) | 131 (60%) | 141 (69%) | |
| Men | 272 (64%) | 146 (66%) | 126 (62%) |
| White | 424 (100%) | 219 (100%) | 205 (100%) |
| BMI (kg/m2) | 27.4 (4.0) | 27.4 (4.1) | 27.3 (4.0) |
| BMI ≥ 30 | 103 (24%) | 53 (24%) | 50 (24%) |
| Smoking | |||
| Never | 198 (47%) | 93 (42%) | 105 (51%) |
| Ex-smoker | 125 (29%) | 64 (29%) | 61 (30%) |
| Current | 100 (24%) | 63 (29%) | 37 (18%) |
| Concomitant disease | |||
| Hypertension | 317 (75%) | 161 (73%) | 156 (76%) |
| Diabetes | 102 (24%) | 51 (23%) | 51 (25%) |
| Hyperlipidaemia | 142 (33%) | 79 (36%) | 63 (31%) |
| Atrial flutter or fibrillation | 16 (4%) | 9 (4%) | 7 (3%) |
| Congestive heart failure | 19 (5%) | 6 (3%) | 13 (6%) |
| History of prior stroke | 61 (14%) | 34 (16%) | 27 (13%) |
| mRS | 2 (0–5) | 2 (0–5) | 2 (0–5) |
| NIHSS | 3 (0–20) | 3 (0–15) | 3 (0–20) |
| Total (n = 425) | Early ASA + ER-DP (n = 220) | Early ASA (n = 205) | p | |
|---|---|---|---|---|
| Baseline | 183 (145–217) | 182 (143–215) | 184 (148–223) | - |
| Day 8 | 186 (156–229) | 186 (154–231) | 186 (158–227) | - |
| Change from baseline | 8 (−25–41) | 9 (−21–41) | 7 (−28–41) | n.s. |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Worthmann, H.; Dengler, R.; Schumacher, H.; Schwartz, A.; Eisert, W.G.; Lichtinghagen, R.; Weissenborn, K. Monocyte Chemotactic Protein-1 as a Potential Biomarker for Early Anti-Thrombotic Therapy after Ischemic Stroke. Int. J. Mol. Sci. 2012, 13, 8670-8678. https://doi.org/10.3390/ijms13078670
Worthmann H, Dengler R, Schumacher H, Schwartz A, Eisert WG, Lichtinghagen R, Weissenborn K. Monocyte Chemotactic Protein-1 as a Potential Biomarker for Early Anti-Thrombotic Therapy after Ischemic Stroke. International Journal of Molecular Sciences. 2012; 13(7):8670-8678. https://doi.org/10.3390/ijms13078670
Chicago/Turabian StyleWorthmann, Hans, Reinhard Dengler, Helmut Schumacher, Andreas Schwartz, Wolfgang G. Eisert, Ralf Lichtinghagen, and Karin Weissenborn. 2012. "Monocyte Chemotactic Protein-1 as a Potential Biomarker for Early Anti-Thrombotic Therapy after Ischemic Stroke" International Journal of Molecular Sciences 13, no. 7: 8670-8678. https://doi.org/10.3390/ijms13078670




