DsHsp90 Is Involved in the Early Response of Dunaliella salina to Environmental Stress †
Abstract
:1. Introduction
2. Results and Discussion
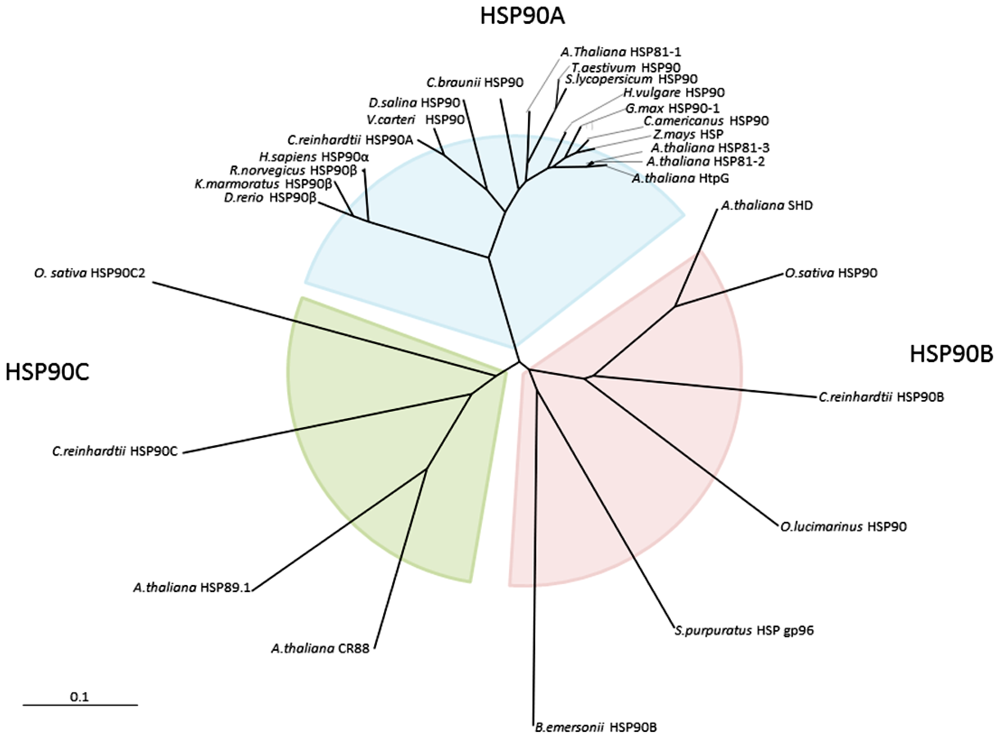
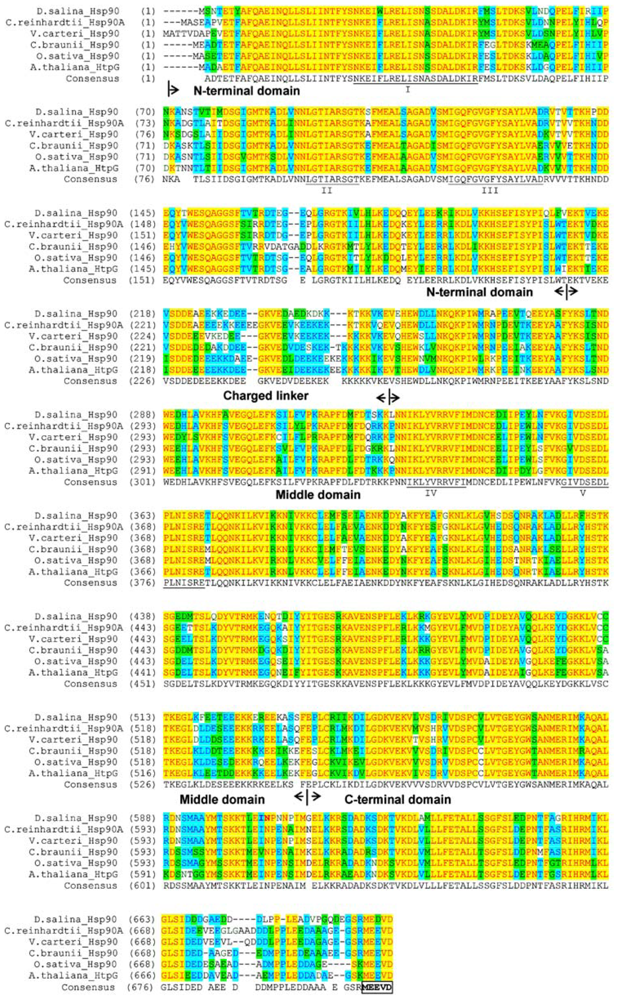
2.1. Cloning and Gene Structure of Dshsp90 from D. Salina
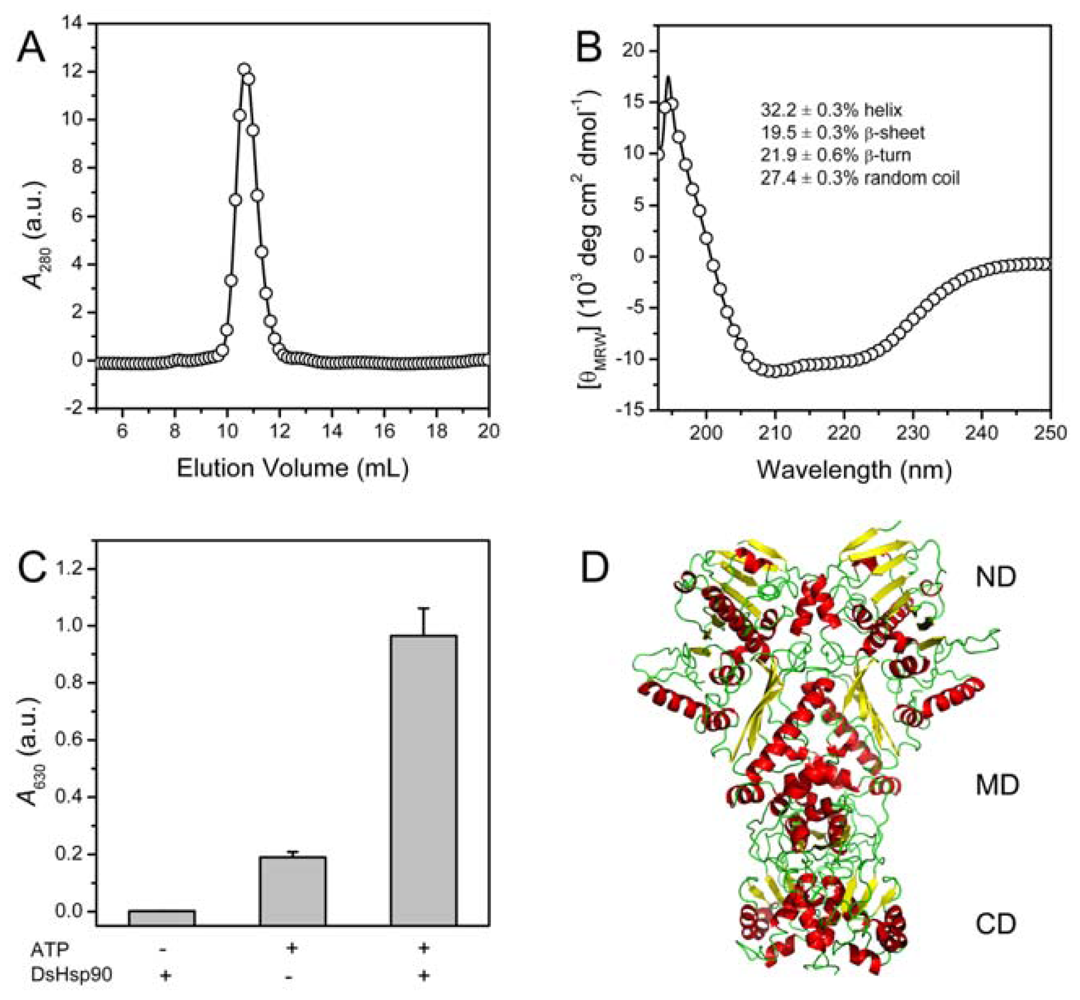
2.2. Biophysical and Biochemical Characterization of Recombinant DsHsp90
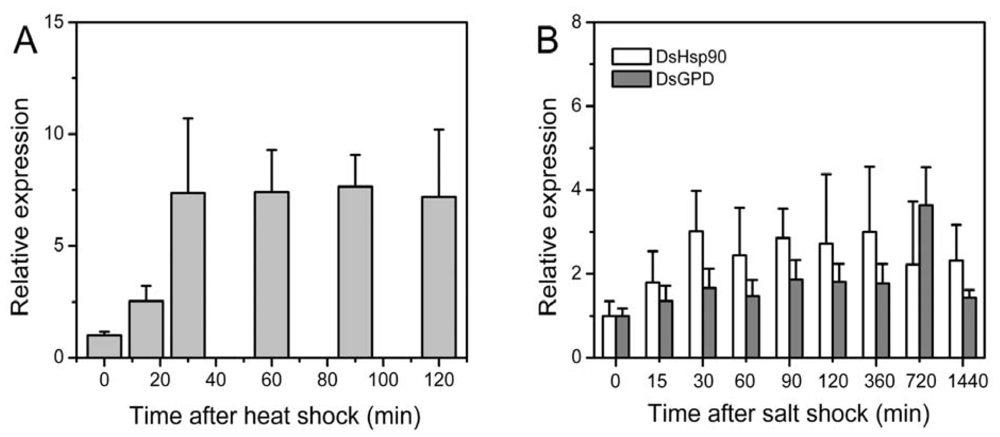
2.3. Relative Expression of the Dshsp90 Gene
3. Experimental Section
3.1. Chemicals
3.2. Algae and Growth Conditions
3.3. Gene Cloning and Sequence Analysis
3.4. Material Treatments and Total RNA Isolation
3.5. Quantitative PCR with SYBR Green
3.6. Protein Expression and Purification
3.7. ATPase Activity Assay
3.8. Spectroscopy
3.9. Homology Modeling
4. Conclusions
Supplementary Materials
ijms-13-07963-s002.pdfAcknowledgements
References
- Oren, A. A hundred years of Dunaliella research: 1905–2005. Saline Syst 2005, 1. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Jiang, J.G. Osmotic responses of Dunaliella to the changes of salinity. J. Cell Physiol 2009, 219, 251–258. [Google Scholar]
- Hosseini Tafreshi, A.; Shariati, M. Dunaliella biotechnology: Methods and applications. J. Appl. Microbiol 2009, 107, 14–35. [Google Scholar]
- Lamers, P.P.; Janssen, M.; de Vos, R.C.; Bino, R.J.; Wijffels, R.H. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol 2008, 26, 631–638. [Google Scholar]
- Ben-Amotz, A.; Shaish, A.; Avron, M. Mode of action of the massively accumulated b-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol 1989, 91, 1040–1043. [Google Scholar]
- Ben-Amotz, A.; Avron, M. The role of glycerol in the osmotic regulation of the halophilic alga Dunaliella parva. Plant Physiol 1973, 51, 875–878. [Google Scholar]
- Borowitzka, L.J.; Brown, A.D. The salt relations of marine and halophilic species of the unicellular green alga, Dunaliella. The role of glycerol as a compatible solute. Arch. Mikrobiol 1974, 96, 37–52. [Google Scholar]
- He, Q.; Qiao, D.; Bai, L.; Zhang, Q.; Yang, W.; Li, Q.; Cao, Y. Cloning and characterization of a plastidic glycerol 3-phosphate dehydrogenase cDNA from Dunaliella salina. J. Plant Physiol 2007, 164, 214–220. [Google Scholar]
- O’Rourke, S.M.; Herskowitz, I.; O’Shea, E.K. Yeast go the whole HOG for the hyperosmotic response. Trends Genet 2002, 18, 405–412. [Google Scholar]
- Jia, Y.; Xue, L.; Li, J.; Liu, H. Isolation and proteomic analysis of the halotolerant alga Dunaliella salina flagella using shotgun strategy. Mol. Biol. Rep 2010, 37, 711–716. [Google Scholar]
- Katz, A.; Waridel, P.; Shevchenko, A.; Pick, U. Salt-induced changes in the plasma membrane proteome of the halotolerant alga Dunaliella salina as revealed by blue native gel electrophoresis and nano-LC-MS/MS analysis. Mol. Cell. Proteomics 2007, 6, 1459–1472. [Google Scholar]
- Liska, A.J.; Shevchenko, A.; Pick, U.; Katz, A. Enhanced photosynthesis and redox energy production contribute to salinity tolerance in Dunaliella as revealed by homology-based proteomics. Plant Physiol 2004, 136, 2806–2817. [Google Scholar]
- Zhao, R.; Cao, Y.; Xu, H.; Lv, L.; Qiao, D.; Cao, Y. Analysis of expressed sequence tags from the green alga Dunaliella salina (chlorophyta). J. Phycol 2011, 47, 1454–1460. [Google Scholar]
- Pearl, L.H.; Prodromou, C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem 2006, 75, 271–294. [Google Scholar]
- Rutherford, S.; Knapp, J.R.; Csermely, P. Hsp90 and developmental networks. Adv. Exp. Med. Biol 2007, 594, 190–197. [Google Scholar]
- Zhao, R.; Houry, W.A. Molecular interaction network of the Hsp90 chaperone system. Adv. Exp. Med. Biol 2007, 594, 27–36. [Google Scholar]
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol 2010, 11, 515–528. [Google Scholar]
- DeZwaan, D.C.; Freeman, B.C. HSP90 manages the ends. Trends Biochem. Sci 2010, 35, 384–391. [Google Scholar]
- Wandinger, S.K.; Richter, K.; Buchner, J. The Hsp90 chaperone machinery. J. Biol. Chem 2008, 283, 18473–18477. [Google Scholar]
- Yang, X.X.; Maurer, K.C.; Molanus, M.; Mager, W.H.; Siderius, M.; van der Vies, S.M. The molecular chaperone Hsp90 is required for high osmotic stress response in Saccharomyces cerevisiae. FEMS Yeast Res 2006, 6, 195–204. [Google Scholar]
- Yang, X.X.; Hawle, P.; Bebelman, J.P.; Meenhuis, A.; Siderius, M.; van der Vies, S.M. Cdc37p is involved in osmoadaptation and controls high osmolarity-induced cross-talk via the MAP kinase Kss1p. FEMS Yeast Res 2007, 7, 796–807. [Google Scholar]
- Li, G.; Liu, M.; Jiang, Y.; Qiao, D.R.; Cao, Y. Construction and functional gene screening of cDNA library of Dunaliella salina. J. Trop. Subtrop. Bot 2004, 12, 74–78. [Google Scholar]
- Gupta, R.S. Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol. Biol. Evol 1995, 12, 1063–1073. [Google Scholar]
- Hawkins, J.D. A survey on intron and exon lengths. Nucleic Acids Res 1988, 16, 9893–9908. [Google Scholar]
- Gilbert, W.; Glynias, M. On the ancient nature of introns. Gene 1993, 135, 137–144. [Google Scholar]
- Rogozin, I.B.; Sverdlov, A.V.; Babenko, V.N.; Koonin, E.V. Analysis of evolution of exon-intron structure of eukaryotic genes. Brief. Bioinform 2005, 6, 118–134. [Google Scholar]
- Roy, S.W.; Gilbert, W. Rates of intron loss and gain: Implications for early eukaryotic evolution. Proc. Natl. Acad. Sci. USA 2005, 102, 5773–5778. [Google Scholar]
- Mourier, T.; Jeffares, D.C. Eukaryotic intron loss. Science 2003, 300. [Google Scholar] [CrossRef]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Marechal-Drouard, L.; et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar]
- Prochnik, S.E.; Umen, J.; Nedelcu, A.M.; Hallmann, A.; Miller, S.M.; Nishii, I.; Ferris, P.; Kuo, A.; Mitros, T.; Fritz-Laylin, L.K.; et al. Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science 2010, 329, 223–226. [Google Scholar]
- Ren, X.Y.; Vorst, O.; Fiers, M.W.; Stiekema, W.J.; Nap, J.P. In plants, highly expressed genes are the least compact. Trends Genet 2006, 22, 528–532. [Google Scholar]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 2002, 30, 325–327. [Google Scholar]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 2005, 10, 88–94. [Google Scholar]
- TFSEARCH: Searching Transcription Factor Binding Sites, version 1.3; Computational Biology Research Center, AIST: Japan, 2002.
- Bastola, D.R.; Pethe, V.V.; Winicov, I. Alfin1, a novel zinc-finger protein in alfalfa roots that binds to promoter elements in the salt-inducible MsPRP2 gene. Plant Mol. Biol 1998, 38, 1123–1135. [Google Scholar]
- Ali, M.M.; Roe, S.M.; Vaughan, C.K.; Meyer, P.; Panaretou, B.; Piper, P.W.; Prodromou, C.; Pearl, L.H. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature 2006, 440, 1013–1017. [Google Scholar]
- Sreerama, N.; Woody, R.W. Estimation of protein secondary structure from circular dichroism spectra: Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem 2000, 287, 252–260. [Google Scholar]
- Itaya, K.; Ui, M. A new micromethod for the colorimetric determination of inorganic phosphate. Clin. Chim. Acta 1966, 14, 361–366. [Google Scholar]
- Brock, T.D. Salinity and the ecology of Dunaliella from great salt lake. J. Gen. Microbiol 1975, 89, 285–292. [Google Scholar]
- Stewart, C.N., Jr; Via, L.E. A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. Biotechniques 1993, 14, 748–750. [Google Scholar]
- Siebert, P.D.; Chenchik, A.; Kellogg, D.E.; Lukyanov, K.A.; Lukyanov, S.A. An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res 1995, 23, 1087–1088. [Google Scholar]
- Noguchi, A.; Takekawa, N.; Einarsdottir, T.; Koura, M.; Noguchi, Y.; Takano, K.; Yamamoto, Y.; Matsuda, J.; Suzuki, O. Chromosomal mapping and zygosity check of transgenes based on flanking genome sequences determined by genomic walking. Exp. Anim 2004, 53, 103–111. [Google Scholar]
- plantCARE. available online: http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ accessed on 27 June 2012.
- Fisher, D.K.; Higgins, T.J. A sensitive, high-volume, colorimetric assay for protein phosphatases. Pharm. Res 1994, 11, 759–763. [Google Scholar]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar]


| Exon No | Exon Size(bp) | Intron Size(bp) | Boundary Sequence | Junction Type | |||
|---|---|---|---|---|---|---|---|
| Exon | 5′-Intron | 3′-Intron | Exon | ||||
| 1 | 98 | 199 | TCTCTG | TGAGTC | TGCAGG | ATCATC | 0 |
| 2 | 43 | 115 | TGCGGG | TGAGTG | CGCAGG | ATGTGA | I |
| 3 | 42 | 206 | TCAGGT | GCGCGC | GCAGGT | TCATGA | I |
| 4 | 56 | 330 | ATCAGG | TCCGTC | TGCAGG | ATCATC | 0 |
| 5 | 64 | 221 | CCAAGG | TCAGTA | TTCAGG | CTGACC | I |
| 6 | 35 | 511 | GCCAGG | TGAGGG | TGCAGG | AGTGGA | 0 |
| 7 | 241 | 307 | TGAAGG | TGTGTG | TGCAGG | AGGACC | II |
| 8 | 144 | 275 | AGAAGG | GTGGGT | AGTCAG | AGGATG | II |
| 9 | 113 | 464 | ATCTGG | TAAGCC | TCCAGG | ATGCGT | I |
| 10 | 124 | 448 | TCAAGT | GCGTGT | GCAGGT | CCATCC | II |
| 11 | 144 | 163 | TCAAGG | TGAGGT | CTGAGG | GTATTG | II |
| 12 | 75 | 289 | AGTTGA | GTAGAA | AGGTGA | TCAAGA | II |
| 13 | 108 | 179 | AGCTGG | TAGGTA | CGCAGG | GTGTGC | II |
| 14 | 150 | 263 | ACTGGT | AAGGAG | TCAGGT | GAGAGC | I |
| 15 | 125 | 707 | TGATGG | TGAGTG | TGCAGG | CAAGAA | I |
| 16 | 137 | 580 | AGGTGG | GCGTCC | AGGTGG | AGAAGG | II |
| 17 | 181 | 277 | TCATGG | TGGGTG | TGCAGG | GTGAGC | II |
| 18 | 51 | 243 | TCAAGG | TAAGGG | TGCAGG | ATCTAG | II |
| 19 | 98 | 108 | ATCAAG | GTAGGT | CTACAG | CTTGGC | 0 |
| 20 | 97 | 373 | TGGAGG | TGAGTC | TGCAGG | ATGTCG | 0 |
| 21 | 180 | ||||||
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, S.-J.; Wu, M.-J.; Chen, X.-J.; Jiang, Y.; Yan, Y.-B. DsHsp90 Is Involved in the Early Response of Dunaliella salina to Environmental Stress †. Int. J. Mol. Sci. 2012, 13, 7963-7979. https://doi.org/10.3390/ijms13077963
Wang S-J, Wu M-J, Chen X-J, Jiang Y, Yan Y-B. DsHsp90 Is Involved in the Early Response of Dunaliella salina to Environmental Stress †. International Journal of Molecular Sciences. 2012; 13(7):7963-7979. https://doi.org/10.3390/ijms13077963
Chicago/Turabian StyleWang, Si-Jia, Ming-Jie Wu, Xiang-Jun Chen, Yan Jiang, and Yong-Bin Yan. 2012. "DsHsp90 Is Involved in the Early Response of Dunaliella salina to Environmental Stress †" International Journal of Molecular Sciences 13, no. 7: 7963-7979. https://doi.org/10.3390/ijms13077963
APA StyleWang, S.-J., Wu, M.-J., Chen, X.-J., Jiang, Y., & Yan, Y.-B. (2012). DsHsp90 Is Involved in the Early Response of Dunaliella salina to Environmental Stress †. International Journal of Molecular Sciences, 13(7), 7963-7979. https://doi.org/10.3390/ijms13077963




