Modification of MCF-10A Cells with Pioglitazone and Serum-Rich Growth Medium Increases Soluble Factors in the Conditioned Medium, Likely Reducing BT-474 Cell Growth
Abstract
:1. Introduction
2. Results and Discussion
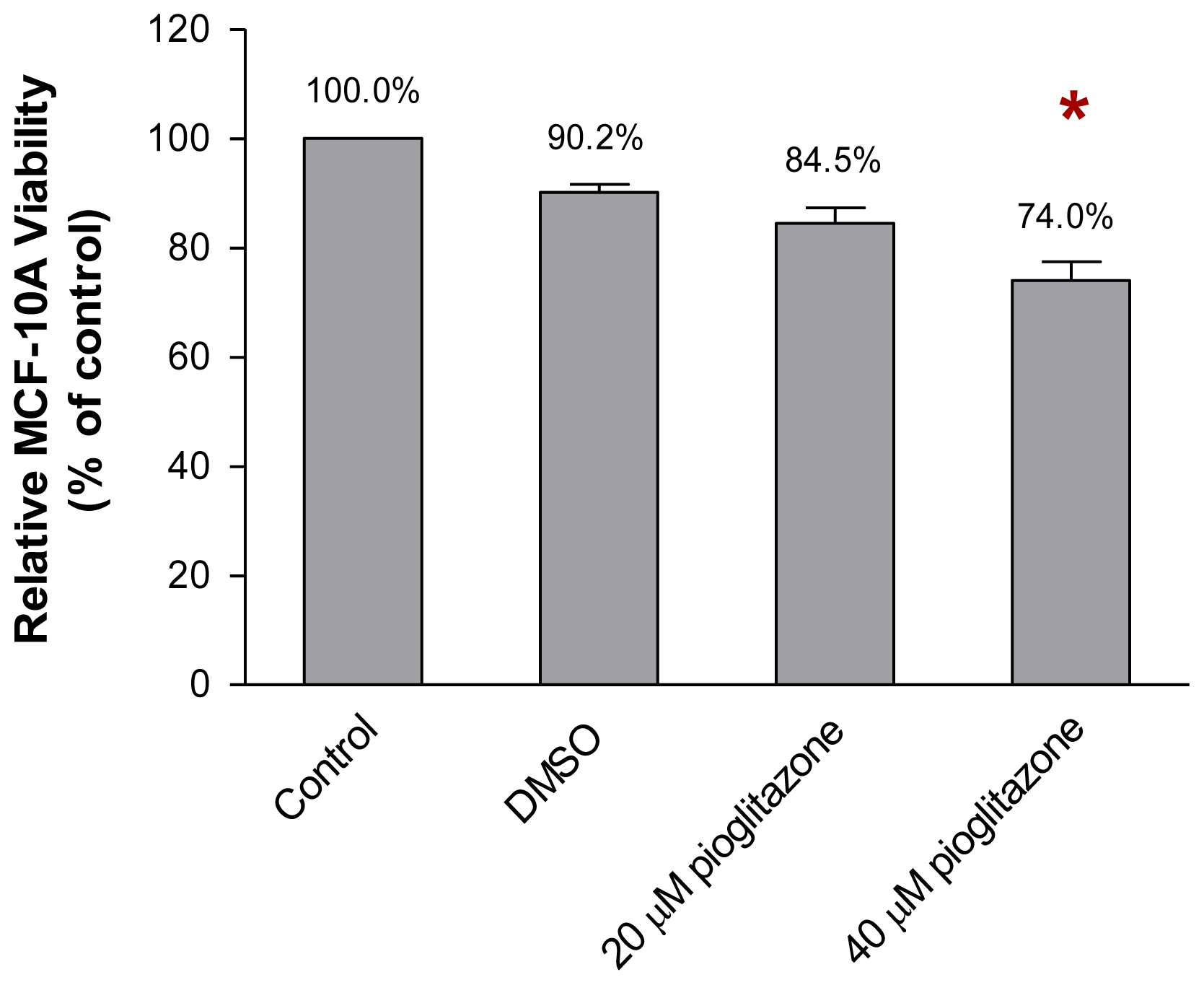
2.1. The Viability of MCF-10A Cells Incubated with Pioglitazone-Containing Growth Medium
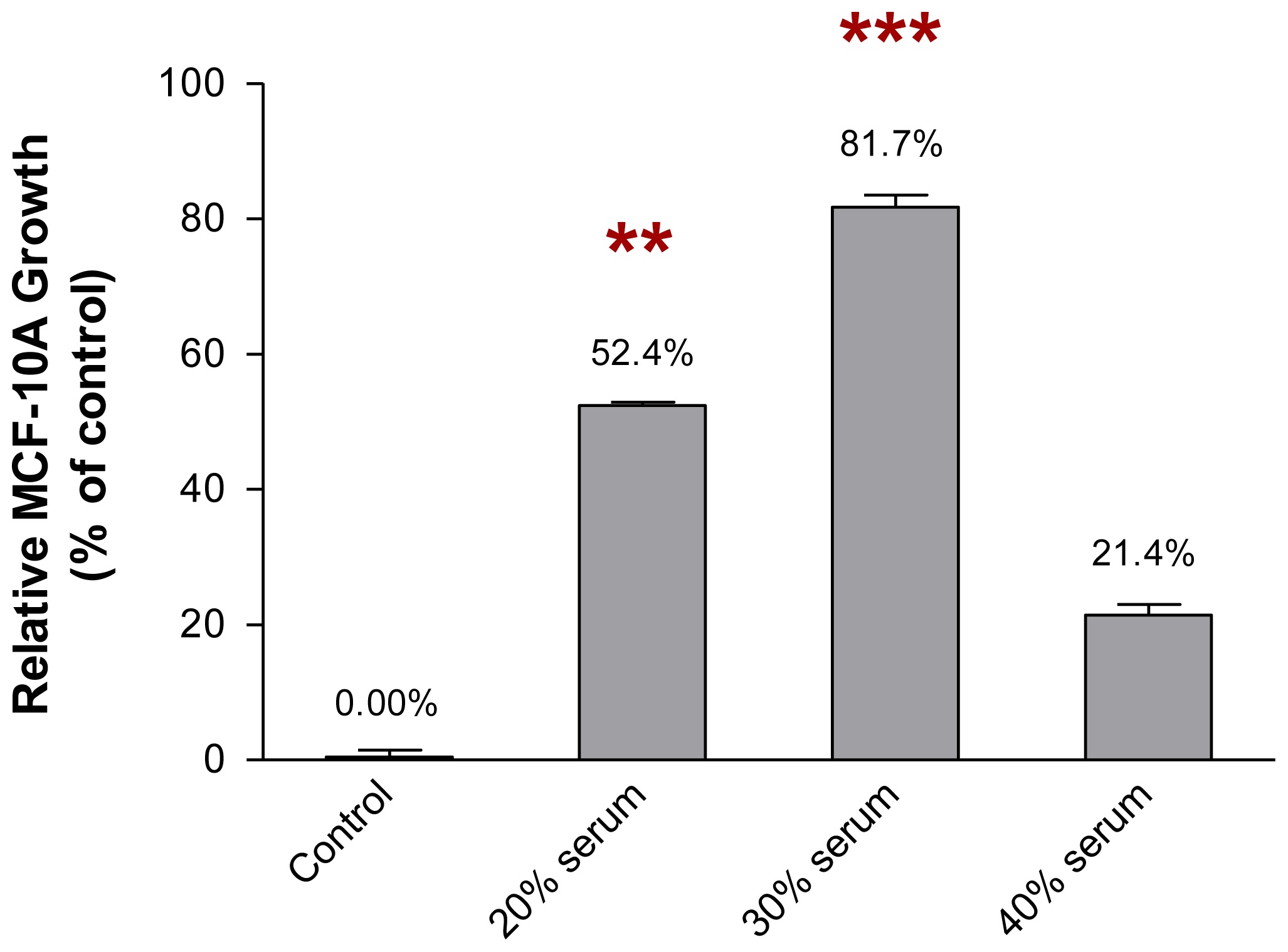
2.2. The Growth of MCF-10A Cells Incubated with Serum-Rich Growth Medium
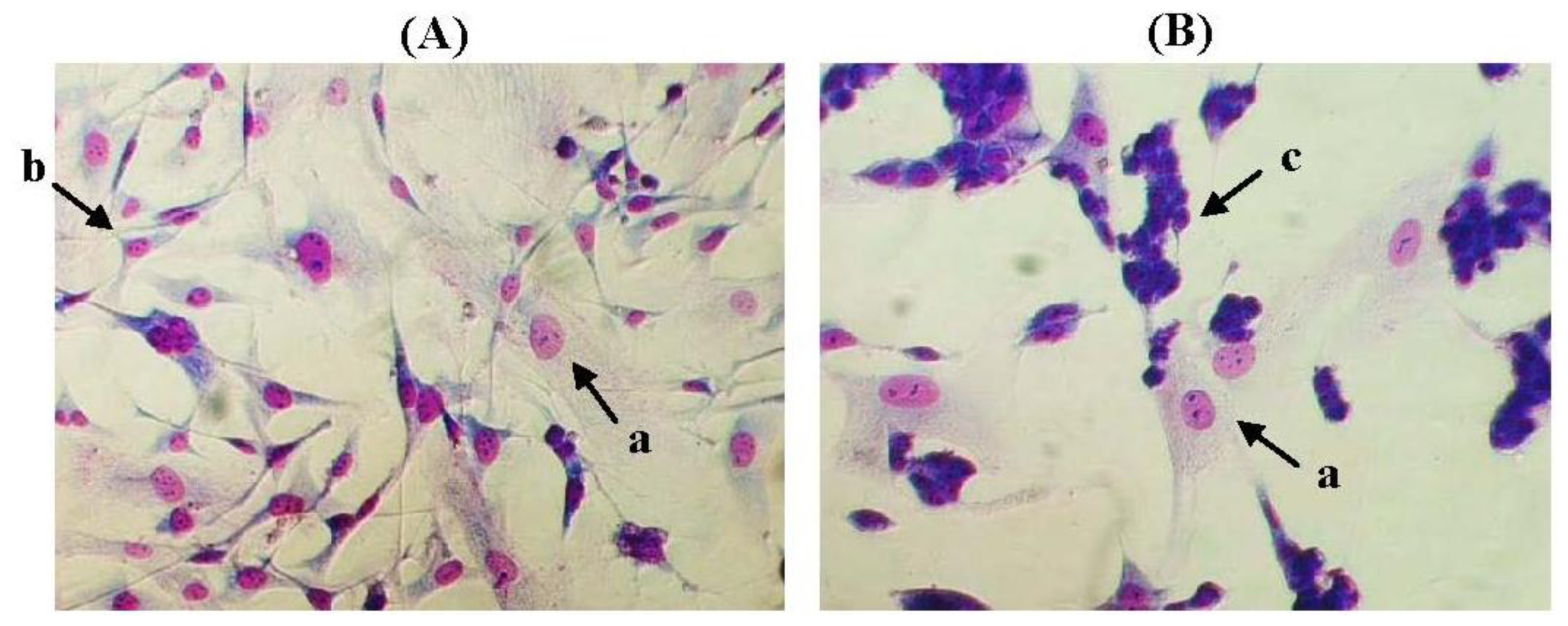
2.3. The Morphology of MCF-10A Cells Preincubated with Pioglitazone and/or Serum-Rich Growth Media
2.4. The Adhesive Interaction of BT-474 and MCF-10A Cells Preincubated with Pioglitazone and/or Serum-Rich Growth Media
2.5. The Non-Adhesive Interaction of BT-474 Cells and MCF-10A Cells Preincubated with Pioglitazone and/or Serum-Rich Growth Media
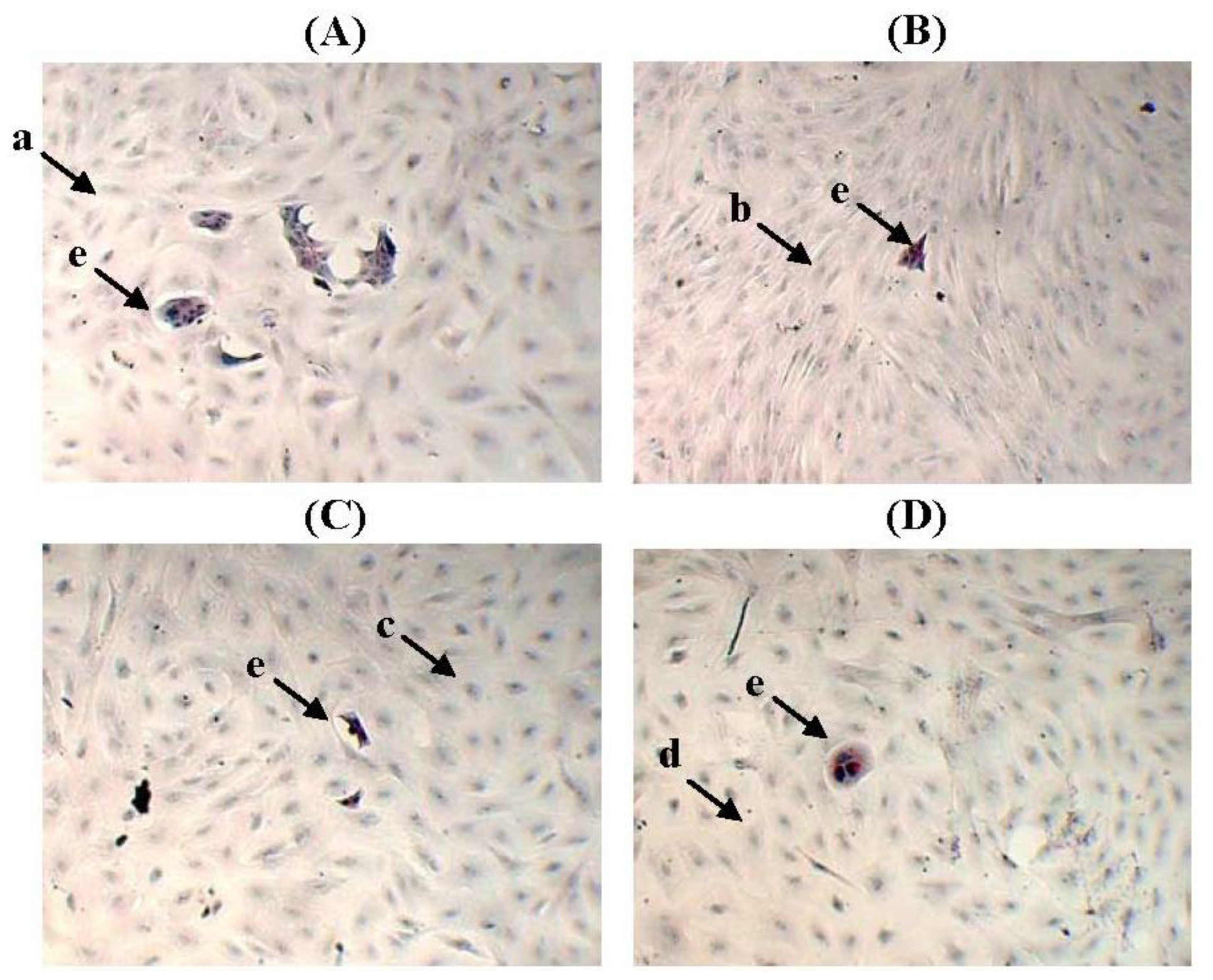
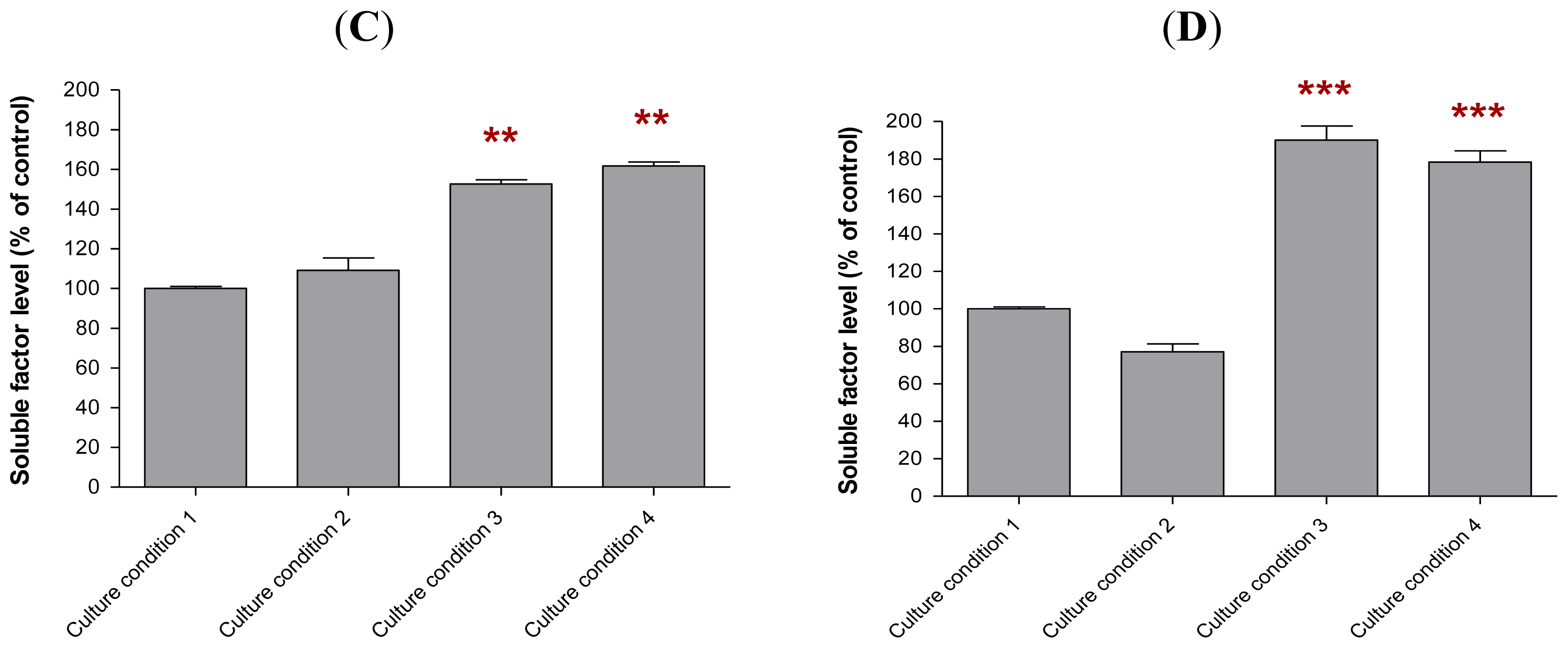
2.6. The Levels of Soluble Factors in the Conditioned Media of Preincubated MCF-10A Cells
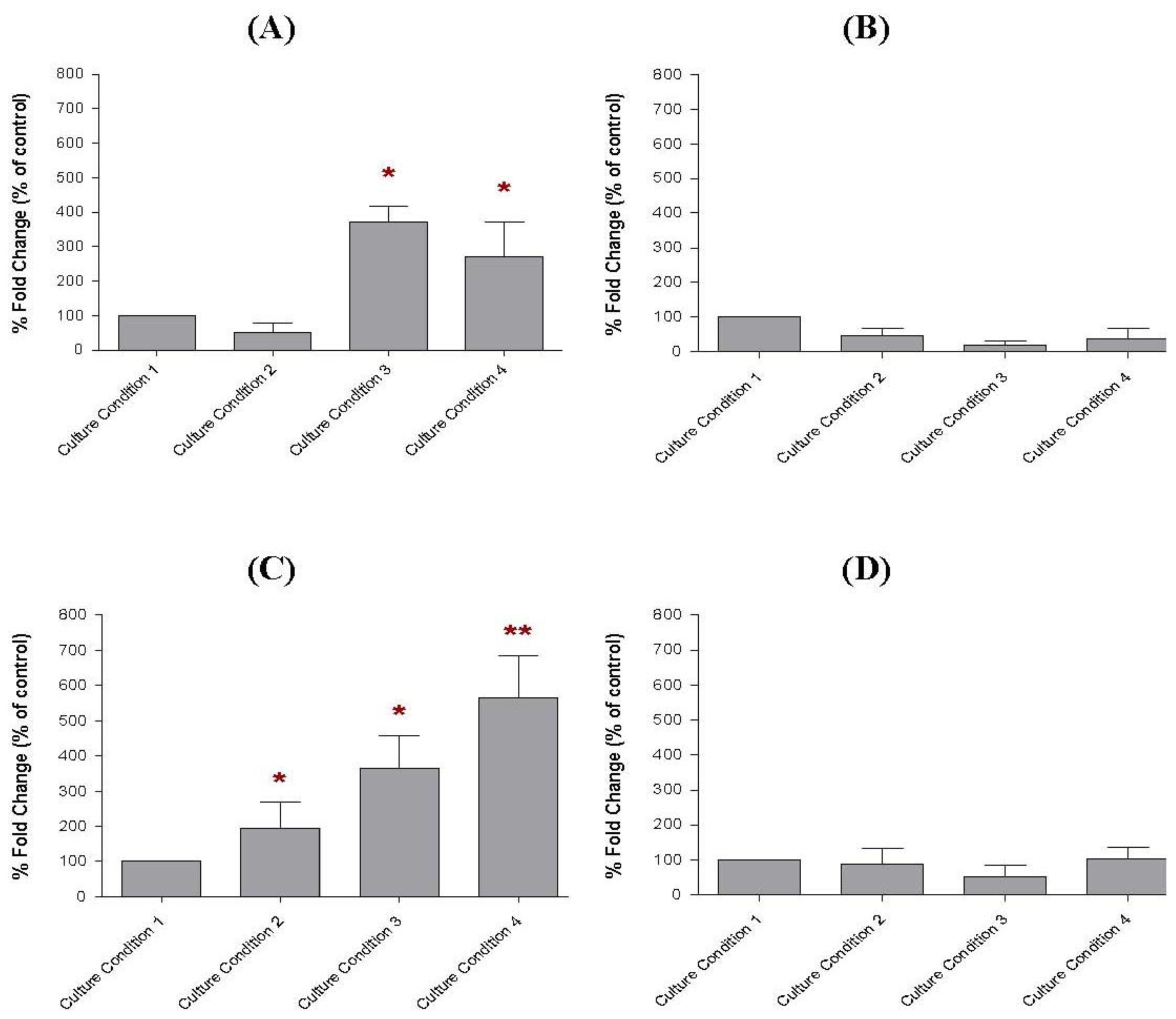
2.7. The Transcript Levels of Soluble Factors in the Preincubated MCF-10A Cells
3. Discussion
4. Experimental Section
4.1. Culturing of MCF-10A and BT-474 Cell Lines
4.2. Viability of MCF-10A Cells Incubated with Pioglitazone-Containing or Serum-Rich Growth Medium
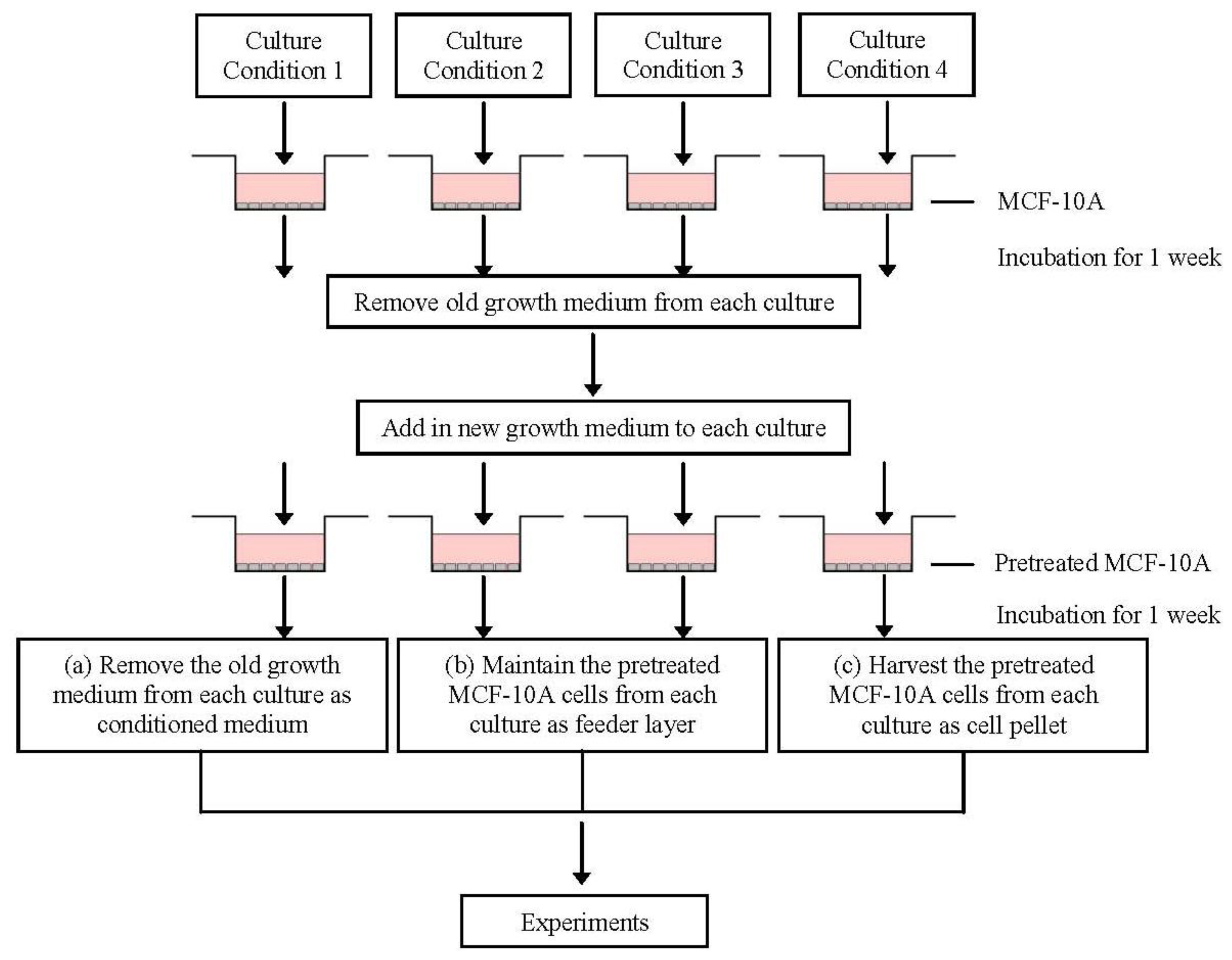
4.3. Preparation of the Conditioned Media and MCF-10A Cells Preincubated with Pioglitazone and/or Serum-Rich Growth Media
4.4. Adhesive Interaction of BT-474 Cells and MCF-10A Cells Preincubated with Pioglitazone and/or Serum-Rich Growth Media
4.5. Non-Adhesive Interaction of BT-474 Cells with MCF-10A Cells Preincubated with Pioglitazone and/or Serum-Rich Growth Media
4.6. Immunoassay of Soluble Factors in the Conditioned Media
4.7. Analysis of Soluble Factor Transcript Expressions in the Preincubated MCF-10A Cells
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
References
- Liu, H.; Zang, C.; Fenner, M.H.; Possinger, K.; Elstner, E. PPARgamma ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res. Treat 2003, 79, 63–74. [Google Scholar]
- Fenner, M.H.; Elstner, E. Peroxisome proliferator-activated receptor-gamma ligands for the treatment of breast cancer. Expert. Opin. Inv. Drug 2005, 14, 557–568. [Google Scholar]
- Mody, M.; Dharker, N.; Bloomston, M.; Wang, P.S.; Chou, F.S.; Glickman, T.S.; McCaffrey, T.; Yang, Z.; Pumfery, A.; Lee, D.; et al. Rosiglitazone sensitizes MDA-MB-231 breast cancer cells to anti-tumour effects of tumour necrosis factor-alpha, CH11 and CYC202. Endocr.-Relat. Cancer 2007, 14, 305–315. [Google Scholar]
- Zhou, J.; Zhang, W.; Liang, B.; Casimiro, M.C.; Whitaker-Menezes, D.; Wang, M.; Lisanti, M.P.; Lanza-Jacoby, S.; Pestell, R.G.; Wang, C. PPARgamma activation induces autophagy in breast cancer cells. Int. J. Biochem. Cell B 2009, 41, 2334–2342. [Google Scholar]
- Fritah, A.; Saucier, C.; de Wever, O.; Bracke, M.; Bieche, I.; Lidereau, R.; Gespach, C.; Drouot, S.; Redeuilh, G.; Sabbah, M. Role of WISP-2/CCN5 in the maintenance of a differentiated and noninvasive phenotype in human breast cancer cells. Mol. Cell Biol 2008, 28, 1114–1123. [Google Scholar]
- Adams, J.; Carder, P.J.; Downey, S.; Forbes, M.A.; MacLennan, K.; Allgar, V.; Kaufman, S.; Hallam, S.; Bicknell, R.; Walker, J.J.; et al. Vascular endothelial growth factor (VEGF) in breast cancer: Comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res 2000, 60, 2898–2905. [Google Scholar]
- Robinson, S.C.; Scott, K.A.; Wilson, J.L.; Thompson, R.G.; Proudfoot, A.E.; Balkwill, F.R. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res 2003, 63, 8360–8365. [Google Scholar]
- Yaal-Hahoshen, N.; Shina, S.; Leider-Trejo, L.; Barnea, I.; Shabtai, E.L.; Azenshtein, E.; Greenberg, I.; Keydar, I.; Ben-Baruch, A. The chemokine CCL5 as a potential prognostic factor predicting disease progression in stage II breast cancer patients. Clin. Cancer Res 2006, 12, 4474–4480. [Google Scholar]
- Vaday, G.G.; Peehl, D.M.; Kadam, P.A.; Lawrence, D.M. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate 2006, 66, 124–134. [Google Scholar]
- Loberg, R.D.; Ying, C.; Craig, M.; Yan, L.; Snyder, L.A.; Pienta, K.J. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia 2007, 9, 556–562. [Google Scholar]
- Ghosh, S.; Sullivan, C.A.; Zerkowski, M.P.; Molinaro, A.M.; Rimm, D.L.; Camp, R.L.; Chung, G.G. High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum. Pathol 2008, 39, 1835–1843. [Google Scholar]
- Hartmann, M.C.; Dwyer, R.M.; Costello, M.; Potter, S.M.; Curran, C.; Hennessy, E.; Newell, J.; Griffin, D.G.; Kerin, M.J. Relationship between CCL5 and transforming growth factor-β1 (TGFβ1) in breast cancer. Eur. J. Cancer 2011, 47, 1669–1675. [Google Scholar]
- Liu, S.; Ginestier, C.; Ou, S.J.; Clouthier, S.G.; Patel, S.H.; Monville, F.; Korkaya, H.; Heath, A.; Dutcher, J.; Kleer, C.G.; et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res 2011, 71, 614–624. [Google Scholar]
- Lavergne, E.; Combadiere, C.; Iga, M.; Boissonnas, A.; Bonduelle, O.; Maho, M.; Debre, P.; Combadiere, B. Intratumoral CC chemokine ligand 5 overexpression delays tumor growth and increases tumor cell infiltration. J. Immunol 2004, 173, 3755–3762. [Google Scholar]
- Conti, I.; Rollins, B.J. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin. Cancer Biol 2004, 14, 149–154. [Google Scholar]
- Nam, J.S.; Kang, M.J.; Suchar, A.M.; Shimamura, T.; Kohn, E.A.; Michalowska, A.M.; Jordan, V.C.; Hirohashi, S.; Wakefield, L.M. Chemokine (C-C motif) ligand 2 mediates the prometastatic effect of dysadherin in human breast cancer cells. Cancer Res 2006, 66, 7176–7184. [Google Scholar]
- Monti, P.; Leone, B.E.; Marchesi, F.; Balzano, G.; Zerbi, A.; Scaltrini, F.; Pasquali, C.; Calori, G.; Pessi, F.; Sperti, C.; et al. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: Regulation of expression and potential mechanisms of antimalignant activity. Cancer Res 2003, 63, 7451–7461. [Google Scholar]
- Ueno, T.; Toi, M.; Saji, H.; Muta, M.; Bando, H.; Kuroi, K.; Koike, M.; Inadera, H.; Matsushima, K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin. Cancer Res 2000, 6, 3282–3289. [Google Scholar]
- Saji, H.; Koike, M.; Yamori, T.; Saji, S.; Seiki, M.; Matsushima, K.; Toi, M. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer 2001, 92, 1085–1091. [Google Scholar]
- Ohta, M.; Kitadai, Y.; Tanaka, S.; Yoshihara, M.; Yasui, W.; Mukaida, N.; Haruma, K.; Chayama, K. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int. J. Oncol 2003, 22, 773–778. [Google Scholar]
- Zinzalla, G.; Haque, M.R.; Basu, B.P.; Anderson, J.; Kaye, S.L.; Haider, S.; Hasan, F.; Antonow, D.; Essex, S.; Rahman, K.M.; et al. A novel small-molecule inhibitor of IL-6 signaling. Bioorg. Med. Chem. Lett 2010, 20, 7029–7032. [Google Scholar]
- Hodge, D.R.; Hurt, E.M.; Farrar, W.L. The role of IL-6 and STAT3 in inflammation and cancer. Eur. J. Cancer 2005, 41, 2502–2512. [Google Scholar]
- David, F.; Farley, J.; Huang, H.; Lavoie, J.P.; Laverty, S. Cytokine and chemokine gene expression of IL-1β stimulated equine articular chondrocytes. Vet. Surg 2007, 36, 221–227. [Google Scholar]
- Blanchard, F.; Duplomb, L.; Baud’huin, M.; Brounais, B. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev 2009, 20, 19–28. [Google Scholar]
- Ara, T.; Declerck, Y.A. Interleukin-6 in bone metastasis and cancer progression. Eur. J. Cancer 2010, 46, 1223–1231. [Google Scholar]
- Liu, X.; Das, A.M.; Seideman, J.; Griswold, D.; Afuh, C.N.; Kobayashi, T.; Abe, S.; Fang, Q.; Hashimoto, M.; Kim, H.; et al. The CC chemokine ligand 2 (CCL2) mediates fibroblast survival through IL-6. Am. J. Respir. Cell Mol. Biol 2007, 37, 121–128. [Google Scholar]
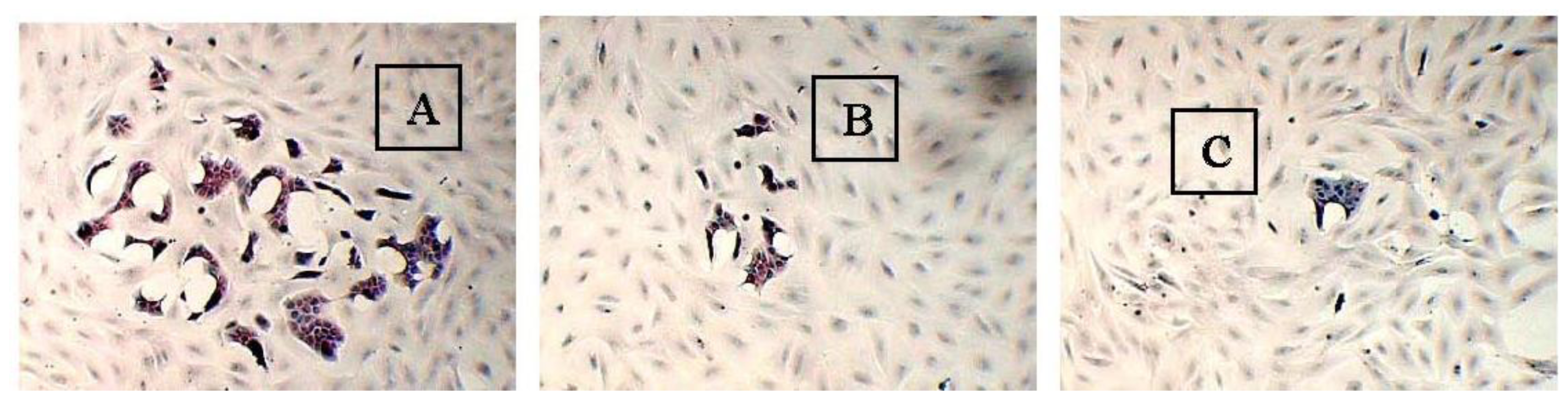
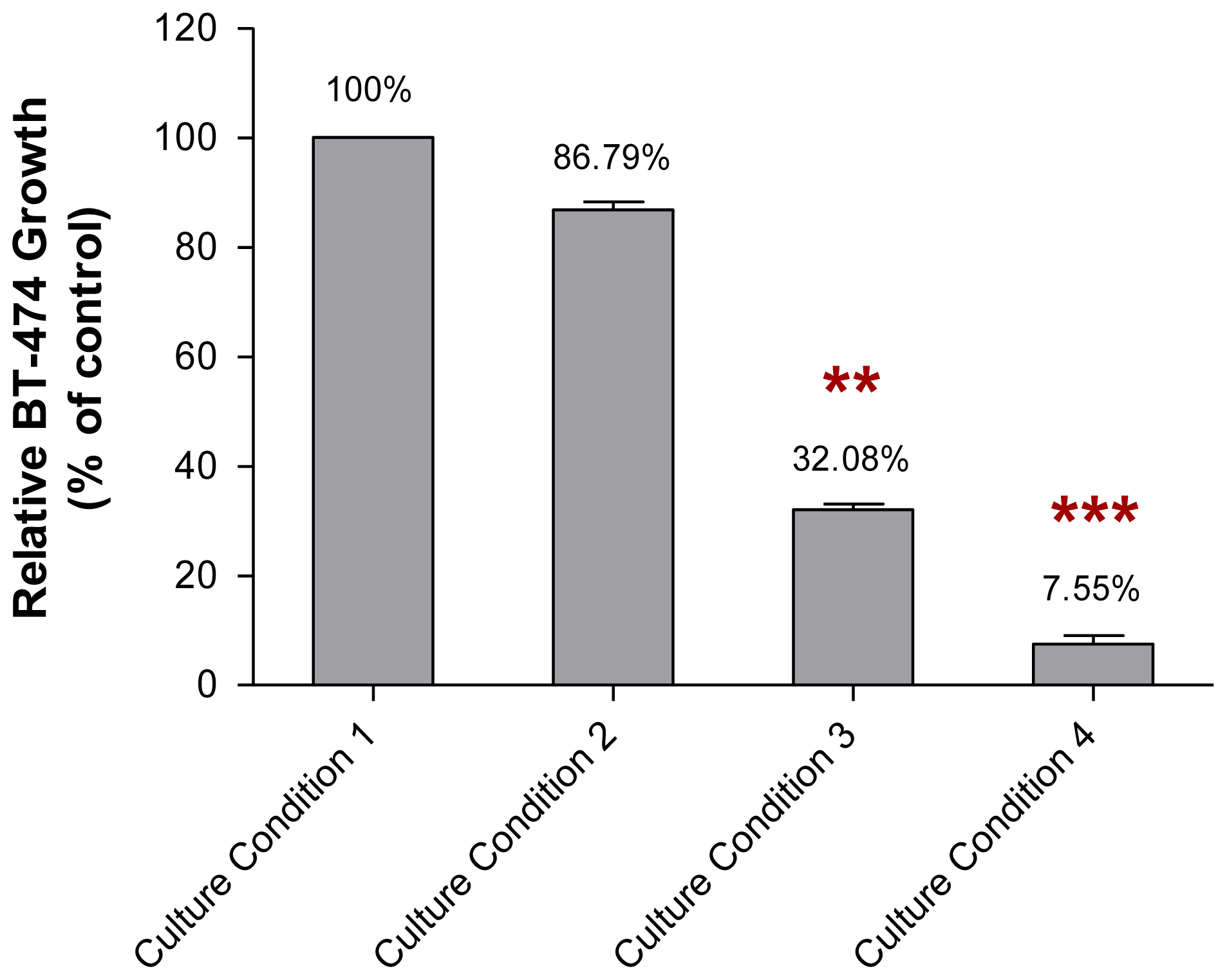


| Culture Condition | Large cell colony | Moderate cell colony | Small cell colony |
|---|---|---|---|
| 1 (control) | 100.0% | - | - |
| 2 | 30.0% | 20.0% | 50.0% |
| 3 | 18.2% | 27.3% | 54.5% |
| 4 | - | 20.0% | 80.0% |
| Culture Condition | Serum Concentration | Pioglitazone Concentration |
|---|---|---|
| 1 (control) | 10% * | - |
| 2 | 30% | - |
| 3 | 10% * | 20 μM |
| 4 | 30% | 20 μM |
| Gene(s) | Sequence(s) | Amplicon (bp) | Tm (°C) |
|---|---|---|---|
| Soluble factor a | Forward: 5′-GCTGTGATCTTCAAGACCATTGTG-3′ | 123 | 80 |
| Reverse: 5′-AGTGAGTGTTCAAGTCTTCGGAGTT-3′ | |||
| Soluble factor b | Forward: 5′-AGCCTCTCCCACAGGTACCAT-3′ | 67 | 85 |
| Reverse: 5′-GGCAGTAGCAATGAGGATGACA-3′ | |||
| Soluble factor c | Forward: CCAGTACCCCCAGGAGAAGATT | 104 | 81 |
| Reverse: CCGTCGAGGATGTACCGAAT | |||
| Soluble factor d | Forward: 5′-CGCAGCTACTGCCATCCAAT-3′ | 81 | 81 |
| Reverse: 5′-TGGCTTGAAGATGTACTCGATCTC-3′ | |||
| β-actin | Forward: 5′-CATTGCCGACAGGATGCA-3′ | 102 | 82 |
| Reverse: 5′-CCGATCCACACGGAGTACTTG-3′ |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Khoo, B.Y.; Miswan, N.; Balaram, P.; Nadarajan, K.; Elstner, E. Modification of MCF-10A Cells with Pioglitazone and Serum-Rich Growth Medium Increases Soluble Factors in the Conditioned Medium, Likely Reducing BT-474 Cell Growth. Int. J. Mol. Sci. 2012, 13, 5607-5627. https://doi.org/10.3390/ijms13055607
Khoo BY, Miswan N, Balaram P, Nadarajan K, Elstner E. Modification of MCF-10A Cells with Pioglitazone and Serum-Rich Growth Medium Increases Soluble Factors in the Conditioned Medium, Likely Reducing BT-474 Cell Growth. International Journal of Molecular Sciences. 2012; 13(5):5607-5627. https://doi.org/10.3390/ijms13055607
Chicago/Turabian StyleKhoo, Boon Yin, Noorizan Miswan, Prabha Balaram, Kalpanah Nadarajan, and Elena Elstner. 2012. "Modification of MCF-10A Cells with Pioglitazone and Serum-Rich Growth Medium Increases Soluble Factors in the Conditioned Medium, Likely Reducing BT-474 Cell Growth" International Journal of Molecular Sciences 13, no. 5: 5607-5627. https://doi.org/10.3390/ijms13055607




