Phenolic Contents and Compositions in Skins of Red Wine Grape Cultivars among Various Genetic Backgrounds and Originations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Anthocyanin Profiles
2.2. Flavonol and Dihydroflavonols Profiles
2.3. Flavan-3-ol Profiles
2.4. Non-Flavonoid Phenolic Profiles
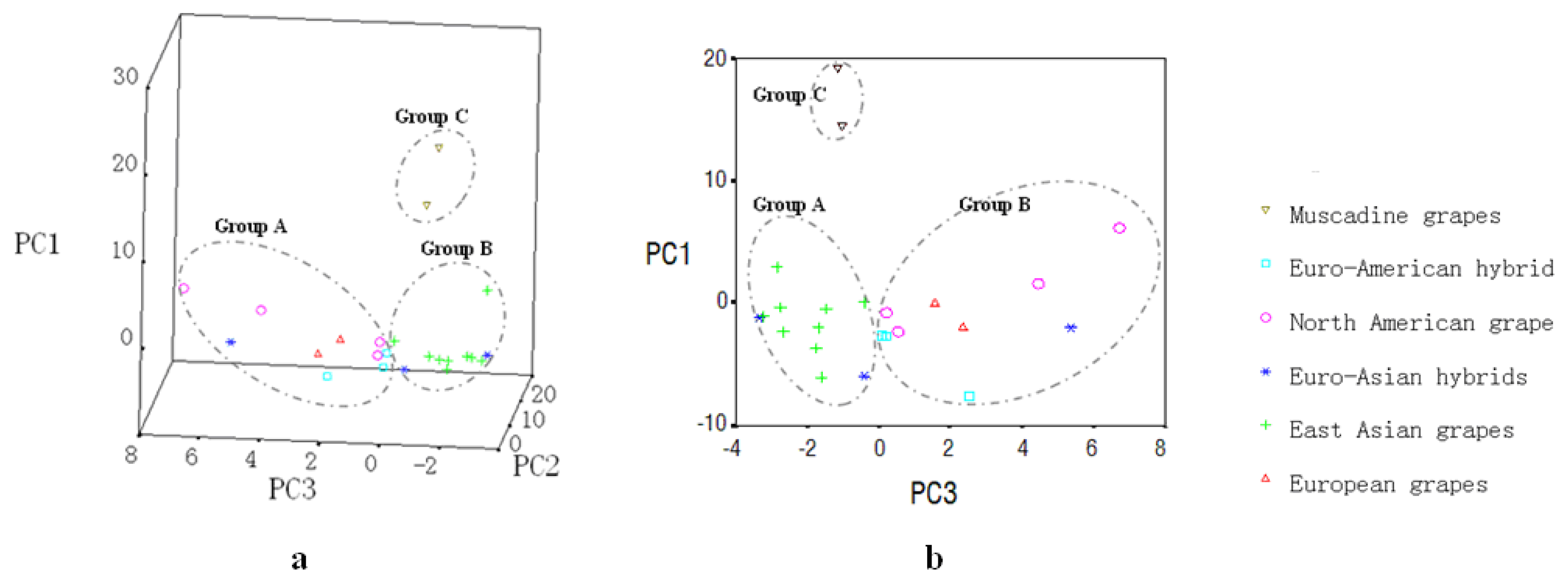
2.5. Principle Component Analysis
3. Experimental Section
3.1. Materials
3.2. Chemicals and Standards
3.3. Extraction of Phenolic Compounds
3.4. Analysis of Phenolic Compounds by HPLC-MS/MS
3.5. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-13-03492-s001.pdfAcknowledgments
References
- Jing, S.X. Grape Classification and Germplasm Resources. In Grape Science, 1st ed; He, P.C., Ed.; China Agricultural Press: Beijing, China, 1999; pp. 8–32. [Google Scholar]
- Kong, Q.-S. Chinese Grape Germplasm, 1st ed; China Agricultural Scientech Press: Beijing, China, 2004; pp. 10–21. [Google Scholar]
- Wang, F.-S.; Zhu, C.-S.; Yang, D.-P.; Chang, H.-T. Systematic study on the genus Vitis L. of China. J. Trop. Subtrop. Botany 2000, 8, 1–10. [Google Scholar]
- Hartle, D.K.; Greenspan, P.; Hargrove, J.L. Muscadine Medicine, 1st ed; Blue Heron Nutraceuticals: Fullerton, CA, USA, 2005; pp. 47–96. [Google Scholar]
- Pezzuto, J.M. Grapes and human health: A perspective. J. Agric. Food Chem 2008, 56, 6777–6784. [Google Scholar]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci 2010, 11, 622–646. [Google Scholar]
- Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J. Food Compos. Anal 2007, 20, 618–626. [Google Scholar]
- Gürbüz, O.; Göçmen, D.; Dađdelen, F.; Gürsoy, M.; Aydin, S.; Şahin, I.; Büyükuysal, L.; Usta, M. Determination of flavan-3-ols and trans-resveratrol in grapes and wine using HPLC with fluorescence detection. Food Chem 2007, 100, 518–525. [Google Scholar]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol profiles of Vitis vinifera white grape cultivars. J. Food Compos. Anal 2010, 23, 699–705. [Google Scholar]
- Sandhu, A.K.; Gu, L.W. Antioxidant capacity, phenolic content, and profiling of phenolic compounds in the seeds, skin, and pulp of Vitis rotundifolia (Muscadine Grapes) as determined by HPLC-DAD- ESI-MS. J. Agric. Food Chem 2010, 58, 4681–4692. [Google Scholar]
- Johnston, T.V.; Morris, J.R. HPLC analysis of Cabernet Sauvignon and noble wine pigment fractions. J. Food Sci 1997, 62, 684–687. [Google Scholar]
- Lee, J.-H.; Johnson, J.V.; Talcott, S.T. Identification of ellagic acid conjugates and other polyphenolics in muscadine grapes by HPLC-ESI-MS. J. Agric. Food Chem 2005, 53, 6003–6010. [Google Scholar]
- Nixdorf, S.L.; Hermosín-Gutiérrez, I. Brazilian red wines made from the hybrid grape cultivar Isabel: Phenolic composition and antioxidant capacity. Anal. Chim. Acta 2010, 659, 208–215. [Google Scholar]
- Lamikanra, O. Anthocyanins of Vitis rotundifolia hybrid grapes. Food Chem 1989, 33, 225–237. [Google Scholar]
- He, J.J. Analysis on Factors of Affecting Anthocyanin Modification in Wine Grapes (Vitis vinifera L.) (in Chinese). Ph.D. Thesis, China Agricultural University, Beijing, China, 2010. [Google Scholar]
- Liang, Z.-H.; Wu, B.-H.; Fan, P.-G.; Yang, C.-X.; Duan, W.; Zheng, X.-B.; Liu, C.-Y.; Li, S.-H. Anthocyanin composition and content in grape berry skin in Vitis germplasm. Food Chem 2008, 111, 837–844. [Google Scholar]
- Huang, Z.-L.; Wang, B.-W.; Williams, P.; Pace, R.D. Identification of anthocyanins in muscadine grapes with HPLC-ESI-MS. LWT–Food Sci. Technol 2009, 42, 819–824. [Google Scholar]
- Dixon, R.A.; Patva, N.L. Stress Induced phenol propanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar]
- Jin, Z.-M.; He, J.-J.; Bi, H.-Q.; Cui, X.-Y.; Duan, C.-Q. Phenolic compound profiles in berry skins from nine red wine grape cultivars in northwest China. Molecules 2009, 14, 4922–4935. [Google Scholar]
- Li, Z.; Pan, Q.-H.; Jin, Z.M.; Mu, L.; Duan, C.-Q. Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in China. Food Chem 2011, 125, 77–83. [Google Scholar]
- Ribichaud, J.L.; Noble, A.C. Astringency and bitterness of selected phenolic in wines. J. Agric. Food Chem 1990, 53, 343–353. [Google Scholar]
- Rodríguez-Montealegre, R.; Romero-Peces, R.; Chacón-Vozmediano, J.L.; Martínez-Gascueña, J.; García-Romero, E. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal 2006, 19, 687–693. [Google Scholar]
- Dopico-García, M.S.; Fique, A.; Guerra, L.; Afonso, J.M.; Pereira, O.; Valentão, P.; Andrade, P.B.; Seabra, R.M. Principal components of phenolics to characterize red Vinho Verde grapes: Anthocyanins or non-coloured compounds? Talanta 2008, 75, 1190–1202. [Google Scholar]
- Hollecker, L.; Pinna, M.; Filippino, G.; Scrugli, S.; Pinna, B.; Argiolas, F.; Murru, M. Simultaneous determination of polyphenolic compounds in red and white grapes grown in Sardinia by high performance liquid chromatography–electron spray ionisation-mass spectrometry. J. Chromatogr. A 2009, 1216, 3402–3408. [Google Scholar]
- Lambert, S.G.; Asenstorfer, R.E.; Williamson, N.M.; Iland, P.G.; Jones, G.P. Copigmentation between malvidin-3-glucoside and some wine constituents and its importance to colour expression in red wine. Food Chem 2011, 125, 106–115. [Google Scholar]
- Xu, C.-M.; Zhang, Y.-L.; Cao, L.; Lu, J. Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chem 2010, 119, 1557–1565. [Google Scholar]
| Grape groups and species | cultivars |
|---|---|
| European grapes | |
| V. vinifera | Cabernet Sauvignon (CS), Merlot (ML) |
| East Asian grapes | |
| V. amurensis | Changbaijiu (CB), Shuanghong (SH), Shuangyou (SY), Zuoshanyi (ZS-1), Zuoshaner (ZS-2) |
| V. dividii | Black Pearl (BP) |
| V.quinquangularis | Mao (MA) |
| V.xunyangensis | Mi (MI) |
| V. ficifolia | Sangye (SN) |
| Euro-Asian hybrids | |
| V. amurensis, V. vinifera | Hasang (HS), Zuohongyi (ZH), Zuoyouhong (ZY) |
| North American grapes | |
| V.aestivalis | Black Spanish (BS) |
| V.labrusca | Catawba (CT), Concord (CC), Niagara Rosada (NR) |
| Euro-American hybrids | |
| V. riparia, V. rupestris, V. vinifera | Marechal Foch (MF) |
| V. rupestris, V. lincecumii, V. vinifera | Chambourcin (CH), St. Croix (SC) |
| Muscadine grapes | |
| V. rotundifolia | Alachua (AL), Noble (NB) |
| Cultivars | Total anthocyanins (TA) | Total flavonols (TFO) | Total flavan-3-ols (TFA) | Total cinnamic acids (TCA) | Total benzoic acid (TBA) | Total ellagic acid (TEA) | Total stilbenes (TS) |
|---|---|---|---|---|---|---|---|
| European grapes | |||||||
| CS | 4163.28 ± 151.49 e,f | 611.75 ± 39.84 g,h,I | 60.89 ± 0.97 a | 1.33 ± 0.00 a | 8.64 ± 0.30 b,c,d,e,f | nd | 26.83 ± 0.86 c |
| ML | 3158.11 ± 323.03 d | 315.83 ± 41.98 b,c,d | 209.84 ± 47.39 a,b,c,d | tr | 10.17 ± 1.95 b,c,d,e,f,g | nd | 167.06 ± 7.21 e |
| East Asian grapes | |||||||
| CB | 4656.70 ± 72.26 g | 438.97 ± 13.61 e,f,g,h | 381.80 ± 12.38 c,d,e | 109.73 ± 2.51 g | 9.49 ± 0.74 b,c,d,e,f,g | nd | tr |
| SH | 6595.13 ± 103.62 i | 199.3 ± 1.75 a,b,c,d | nd | 70.53 ± 2.93 e,f,g | 15.30 ± 0.01 f,g,h,i,j | nd | nd |
| SY | 4479.19 ± 125.29 f,g | 67.08 ± 3.03 a | nd | 78.80 ± 2.57 f,g | 7.92 ± 0.03 b,c,d,e | nd | 3.79 ± 0.45 a |
| ZS-1 | 10036.96 ± 113.72 m | 192.27 ± 2.26 a,b,c,d | nd | 38.08 ± 0.46 a,b,c,d,e | 6.53 ± 1.38 a,b,c | nd | tr |
| ZS-2 | 14370.94 ± 16.04 o | 113.29 ± 0.36 a,b | nd | 61.90 ± 0.97 d,e,f | 11.54 ± 0.93 c,d,e,f,g | nd | tr |
| BP | 1607.43 ± 62.31 b,c | 392.36 ± 0.88 d,e,f,g | 16.46 ± 1.14 a | 216.19 ± 27.17 i | 12.86 ± 1.93 c,d,e,f,g | nd | 2.83 ± 0.37 a |
| MA | 1192.31 ± 85.32 a,b | 385.74 ± 11.02 d,e,f | 16.08 ± 7.05 a | 174.13 ± 1.29 h | 7.09 ± 0.11 a,b,c | nd | 22.37 ± 0.80 b,c |
| MI | 1801.37 ± 64.34 c | 326.26 ± 18.68 b,c,d,e | 14.30 ± 4.83 a | 229.37 ± 7.66 i | 21.70 ± 0.60 j,k,l | nd | 2.17 ± 0.14 a |
| SN | 11580.58 ± 127.70 n | 1358.17 ± 123.03 k | 69.96 ± 2.62 a | 22.68 ± 1.07 a,b,c,d | 27.85 ± 1.28 l | nd | 75.76 ± 0.72 d |
| Euro-Asian hybrids | |||||||
| HS | 8029.36 ± 184.13 k | 613.97 ± 48.46 h,i | 562.73 ± 308.23 e | 55.04 ± 0.49 c,d,e,f | 7.32 ± 3.25 a,b,c,d | nd | 27.31 ± 5.15 c |
| ZH | 13901.93 ± 47.90 o | 359.46 ± 15.11 c,d,e | nd | 49.83 ± 1.18 b,c,d,e,f | 14.19 ± 0.54 d,e,f,g,h | nd | 3.64 ± 0.05 a |
| ZY | 4421.08 ± 78.47 f,g | 241.00 ± 13.68 a,b,c,d,e | nd | 219.42 ± 38.59 I | 15.90 ± 0.35 g,h,i,j | nd | nd |
| North American grapes | |||||||
| BS | 8842.82 ± 18.65 l | 626.81 ± 25.98 h,i | 6.45 ± 0.64 a | 49.43 ± 2.86 b,c,d,e,f | 14.25 ± 3.41 e,f,g,h,i | nd | tr |
| CT | 1457.04 ± 13.47 a,b,c | 277.09 ± 13.61 a,b,c,d,e | 9.66 ± 1.64 a | 28.72 ± 2.88 a,b,c,d | 0.58 ± 0.33 a | nd | nd |
| CC | 5792.99 ± 258.15 h | 1200.41 ± 34.15 j,k | 181.26 ± 3.30 a,b,c | 77.80 ± 0.13 f,g | 12.73 ± 0.14 c,d,e,f,g | nd | nd |
| NR | 1065.63 ± 12.84 a | 1024.86 ± 47.31 j | 1243.67 ± 28.54 f | 20.43 ± 1.01 a,b,c | 3.43 ± 0.92 a,b | nd | nd |
| Euro-American hybrids | |||||||
| MF | 2949.30 ± 87.68 d | 138.70 ± 12.40 a,b,c | 15.85 ± 0.34 a | 13.29 ± 0.14 a,b | 6.08 ± 0.01 a,b,c | nd | tr |
| CH | 8450.64 ± 20.74 k,l | 612.25 ± 6.95 g,h,i | 180.89 ± 15.60 a,b,c | 51.22 ± 3.59 b,c,d,e,f | 14.39 ± 0.13 e,f,g,h,i | nd | tr |
| SC | 16840.99 ± 60.59 p | 376.81 ± 10.77 d,e,f | 55.80 ± 14.96 a | 33.08 ± 6.43 a,b,c,d,e | 14.42 ± 4.35 e,f,g,h,i | nd | nd |
| Muscadine grapes | |||||||
| AL | 2920.11 ± 25.80 d | 1856.99 ± 202.38 l | 350.62 ± 36.67 b,c,d,e | 57.98 ± 6.81 c,d,e,f | 14.03 ± 0.91 d,e,f,g,h | 525.16 ± 123.42a | 12.15 ± 4.17 a,b |
| NB | 5548.91 ± 6.36 h | 1892.53 ± 53.60 l | 451.48 ± 48.98 d,e | 24.89 ± 2.53 a,b,c,d | 21.09 ± 3.56 i,j,k,l | 647.68 ± 148.81 b | 71.67 ± 12.51 d |
| Compounds | Rt (min) | MS; MS2 (m/z) | Eu-grapes | As-grapes | Eu-As hybrids | Am-grapes | Eu-Am hybrids | Mu-grapes |
|---|---|---|---|---|---|---|---|---|
| Dp-3,5-diglc | 3.45 | 627;465,303 | nd | 896.43 (0–2378.51) | 562.78 (106.45–839.73) | 214.45 (0–644.79) | 440.01 (89.48–1038.96) | 926.30 (533.73–1318.86) |
| Cy-3,5-diglc | 3.54 | 611;449,287 | nd | 415.83 (0–1143.16) | 263.13 (14.46–452.11) | 166.45 (83.65–272.07) | 206.92 (29.23–529.53) | 1285.33 (563.84–2006.81) |
| Pt-3,5-diglc | 3.80 | 641;479,317 | nd | 858.62 (33.51–2734.42) | 315.21 (33.73–474.79) | 156.89 (0–499.54) | 596.19 (35.66–1426.36) | 895.45 (653.75–1137.16) |
| Dp-3-glc | 4.43 | 465;303 | 123.39 (123.18–123.60) | 606.35 (0–2634.50) | 2968.24 (505.87–5679.48) | 600.33 (141.58–1137.04) | 1156.07 (343.65–2383.92) | nd |
| Pg-3,5-diglc | 4.63 | 595;433,271 | nd | nd | nd | nd | nd | 97.18 (31.59–162.77) |
| Pn-3,5-diglc | 5.33 | 625;463, 301 | nd | 416.21 (37.87–897.11) | 254.24 (41.84–429.82) | 195.07 (0–568.77) | 262.11 (36.16–498.54) | 678.16 (190.00–1166.33) |
| Mv-3,5-diglc | 5.73 | 655;493, 331 | nd | 2371.40 (133.01–4663.20) | 1366.12 (53.16–2547.98) | 367.52 (0–1277.69) | 1940.85 (268.32–2950.67) | 352.08 (162.06–542.11) |
| Cy-3-glc | 6.25 | 449;287 | 47.79 (47.21–48.37) | nd | 197.68 (0–593.03) | 508.30 (40.07–741.42) | nd | nd |
| Pt-3-glc | 7.33 | 479;317 | 122.43 (116.90–127.97) | 194.89 (0–748.27) | 1266.11 (96.60–2153.69) | 136.83 (6.12–252.17) | 800.43 (404.02–1105.79) | nd |
| Pg-3-glc | 8.70 | 433;271 | nd | nd | nd | 14.24 (0–47.45) | nd | nd |
| Cy-3-acglc-5-glc | 8.96 | 653;611,449,287 | nd | 0 (0-tr) | nd | nd | 2.36 (0–7.08) | nd |
| Pt-3-acglc-5-glc | 9.28 | 683;641,479,317 | nd | nd | nd | 15.60 (0–62.39) | 46.71 (0–140.13) | nd |
| Pn-3-glc | 10.02 | 463;301 | 241.23 (218.63–263.83) | 51.48 (0–272.50) | 122.68 (29.55–268.92) | 71.01 (12.70–134.85) | 79.60 (39.56–106.07) | nd |
| Mv-3-glc | 11.01 | 493;331 | 1794.77 (1436.27–2153.27) | 202.71 (27.38–967.11) | 655.32 (95.39–1458.67) | 78.12 (0–201.12) | 1094.53 (488.69–1634.41) | nd |
| Dp-3-cfglc-5-glc | 11.48 | 789;627,465,303 | nd | nd | nd | 19.95 (0–79.81) | nd | nd |
| Dp-3-cis-cmglc-5-glc | 12.07 | 773;611,465,303 | nd | nd | nd | 30.37 (0–91.26) | nd | nd |
| Pn-3-acglc-5-glc | 12.30 | 667;625,463,301 | nd | nd | nd | nd | nd | nd |
| Dp-3-acglc | 12.50 | 507;465,303 | nd | nd | nd | 18.29 (0–73.15) | 85.36 (34.04–169.32) | nd |
| Mv-3-acglc-5-glc | 12.90 | 697;655,493,331 | nd | 4.35 (0–24.29) | nd | 15.17 (0–60.69) | 53.20 (27.98–91.20) | nd |
| Dp-3-trans-cmglc-5-glc | 14.55 | 773;611,465,303 | nd | 4.38 (0–16.54) | 75.36 (0–226.08) | 405.99 (0–1304.32) | 605.56 (18.45–1666.21) | nd |
| Cy-3-acglc | 14.73 | 491;449,287 | nd | nd | nd | 10.54 (0–42.15) | nd | nd |
| Dp-3-cfglc | 14.96 | 627;465,303 | nd | 0.67 (0–6.07) | 21.86 (0–65.57) | nd | nd | nd |
| Pt-3-cis-cmglc-5-glc | 15.49 | 787;625,479,317 | nd | 0.81 (0–7.30) | 4.97 (0–14.90) | 15.98 (0–55.27) | 8.87 (0–26.62) | nd |
| Pt-3-acglc | 16.22 | 521;317 | nd | 0 (1-tr) | 21.86 (0–65.58) | 22.68 (0–45.40) | 52.70 (35.50–77.05) | nd |
| Dp-3-cis-cmglc | 17.22 | 611;303 | nd | 1.10 (0–9.86) | nd | nd | nd | nd |
| Cy-3-cmglc-5-glc | 17.58 | 757;595,449,287 | nd | nd | 20.35 (0–61.05) | 166.05 (0–336.70) | 101.12 (0–289.80) | nd |
| Pt-3-trans-cmglc-5-glc | 17.78 | 787;625,479,317 | nd | 6.95 (0–40.05) | 59.08 (0–177.25) | 206.07 (0–691.57) | 404.10 (46.92–973.12) | nd |
| Mv-3-cfglc-5-glc | 18.22 | 817;655,493,331 | nd | 10.70 (0–79.97) | nd | nd | nd | nd |
| Pt-3-cfglc | 19.01 | 641;479,317 | nd | 1.24 (0–11.20) | nd | nd | 16.90 (10.43–24.90) | nd |
| Pn-3-cis-cmglc-5-glc | 19.23 | 771;609,463,301 | nd | 0 (0-tr) | nd | 8.45 (0–21.84) | nd | nd |
| Mv-3-cis-cmglc-5-glc | 19.56 | 801;639,493,331 | nd | 6.87 (0–24.03) | nd | nd | 16.08 (0–48.24) | nd |
| Dp-3-trans-cmglc | 20.11 | 611;303 | nd | 8.99 (0–67.26) | 390.23 (0–1170.68) | 319.51 (7.03–798.26) | 575.88 (38.37–1456.98) | nd |
| Pn-3-acglc | 20.33 | 505;301 | 55.85 (51.03–60.67) | nd | nd | 23.02 (0–92.07) | 3.55 (0–10.65) | nd |
| Mv-3-acglc | 20.87 | 535;331 | 511.05 (414.43–607.68) | 2.06 (0–18.51) | 10.02 (0–30.07) | 12.44 (0–33.86) | 52.88 (15.20–114.01) | nd |
| Cy-3-cis-cmglc | 21.03 | 595;287 | nd | nd | nd | 5.34 (0–13.49) | nd | nd |
| Pn-3-trans-cmglc-5-glc | 21.35 | 771;609,463,301 | nd | nd | nd | 104.79 (0–328.97) | nd | nd |
| Mv-3-trans-cmglc-5-glc | 21.69 | 801;639,493,331 | nd | 139.26 (0–583.23) | 18.80 (0–56.41) | 123.78 (0–391.45) | 348.75 (45.62–702.93) | nd |
| Pt-3-cis-cmglc | 22.02 | 625;317 | nd | 0.93 (0–8.36) | nd | nd | nd | nd |
| Mv-3-cfglc | 23.41 | 655;331 | 38.05 (26.26–49.85) | 1.44 (0–12.96) | 47.97 (0–143.90) | 158.01 (36.06–454.79) | 65.05 (10.99–157.78) | nd |
| Cy-3-trans-cmglc | 24.67 | 595;287 | nd | 1.82 (0–16.38) | 2.58 (0–7.74) | nd | 19.96 (0–39.02) | nd |
| Pt-trans-cmglc | 25.84 | 625;317 | 72.60 (69.43–75.78) | 9.50 (0–75.70) | 114.79 (0–344.38) | 56.37 (0–132.89) | 191.91 (28.41–346.48) | nd |
| Pn-3-cis-cmglc | 26.50 | 609;301 | nd | 0 (0-tr) | nd | nd | nd | nd |
| Mv-3-cis-cmglc | 27.60 | 639;331 | nd | 2.46 (0–22.12) | nd | nd | 4.37 (0–13.12) | nd |
| Pn-3-trans-cmglc | 29.23 | 609;301 | 98.17 (93.57–102.76) | 25.26 (0–219.00) | 3.66 (0–10.97) | 16.27 (0–37.45) | 10.95 (7.98–13.14) | nd |
| Mv-3-trans-cmglc | 29.66 | 639;331 | 555.35 (544.50–566.21) | 15.12 (0–70.23) | 21.08 (0–63.23) | 25.74 (0–55.25) | 170.65 (49.41–313.01) | nd |
| Compounds | Rt (min) | MS; MS2 (m/z) | Eu-grapes | As-grapes | Eu-As hybrids | Am-grapes | Eu-Am hybrids | Mu-grapes |
|---|---|---|---|---|---|---|---|---|
| Dihydroflavonols and flavonols (μg QE/g DW) | ||||||||
| Dk-3-glc | 5.00 | 449;287 | nd | nd | nd | nd | nd | 66.68 (0–133.35) |
| K-3-hex | 5.98 | 447;285 | nd | nd | nd | 32.47 (0–53.11) | nd | nd |
| Q-3-hex | 7.37 | 463;301 | nd | nd | 3.96 (0–11.89) | 17.04 (0–28.70) | 5.35 (0–16.05) | 30.80 (18.40–43.20) |
| M-3-gcn | 8.36 | 493;317 | nd | 2.99 (0–26.94) | nd | nd | nd | nd |
| Dq-3-hex | 9.50 | 465;303 | 14.69 (0–29.37) | 2.74 (0–13.82) | 5.76 (0–17.27) | 6.32 (0–12.80) | 2.96 (0–8.87) | nd |
| M-3-gal | 12.44 | 479;317 | nd | 6.22 (0–55.95) | 4.88 (0–14.63) | 2.83 (0–11.34) | nd | nd |
| M-3-glc | 13.45 | 479,317 | 20.37 (19.78–20.96) | 28.38 (0–88.99) | 36.00 (29.31–47.20) | 8.02 (0–19.58) | 13.15 (0–20.50) | 34.31 (30.12–38.50) |
| M-3-rha | 16.34 | 463;317 | nd | 6.32 (0–38.61) | nd | 9.43 (0–37.72) | nd | 215.85 (193.38–238.31) |
| Q | 16.61 | 301 | nd | 36.24 (0–147.55) | 54.38 (0–129.12) | 23.82 (0–95.29) | 36.13 (0–69.21) | 505.02 (452.60–557.43) |
| Dq-3-rha | 17.70 | 449;303 | 53.46 (30.23–76.70) | nd | nd | 19.83 (0–69.93) | nd | nd |
| Q-3-gal | 18.14 | 463;301 | 19.00 (11.94–26.06) | 7.62 (0–39.09) | 6.50 (0–19.49) | 4.10 (0–16.40) | 6.49 (0–19.46) | nd |
| Q-3-gcn | 19.23 | 477;301 | 39.82 (33.32–46.33) | 60.26 (11.84–166.95) | 77.76 (37.42–141.04) | 154.05 (88.97–212.12) | 121.33 (35.54–272.54) | nd |
| Q-3-glc | 19.79 | 463;301 | 85.86 (56.66–115.06) | 57.65 (16.92–155.21) | 50.19 (25.75–85.48) | 170.01 (68.01–369.49) | 69.81 (7.71–166.18) | nd |
| Q-3-rut | 20.03 | 609;301 | nd | 2.06 (0–18.58) | 74.33 (0–223.00) | 74.61 (0–278.92) | 39.66 (0–100.95) | nd |
| Q-3-rha | 20.84 | 447,301 | nd | 111.70 (0–528.65) | nd | 66.07 (0–176.20) | 5.04 (0–15.13) | 652.76 (630.48–675.03) |
| L-3-glc | 21.92 | 493;331 | 20.04 (16.55–23.53) | 3.85 (0–15.87) | 6.34 (0–19.02) | 3.30 (0–13.21) | 8.07 (0–12.96) | nd |
| K-3-gal | 22.27 | 447;285 | 6.83 (0–13.67) | nd | nd | nd | nd | 13.33 (0–26.66) |
| Q-3-xyl | 22.30 | 433;301 | nd | 12.49 (0–47.22) | 13.88 (0–41.65) | 8.49 (0–33.98) | 14.17 (0–26.25) | 244.04 (240.55–247.53) |
| I-3-xyl | 22.57 | 447;315 | nd | 2.67 (0–24.03) | nd | 10.05 (0–40.20) | nd | 14.15 (0–28.30) |
| K-3-rha | 22.74 | 431;285 | nd | 8.17 (0–57.18) | nd | nd | nd | 63.87 (63.09–64.65) |
| Dk-3-rha | 24.28 | 433;287 | nd | 2.96 (0–26.68) | nd | 4.43 (0–17.70) | nd | nd |
| K-3-glc | 25.58 | 447;285 | 51.34 (0–102.68) | nd | nd | nd | nd | 20.89 (15.69–26.09) |
| L-3-acglc | 28.13 | 535;331 | nd | 12.45 (0–62.68) | 8.13 (0–24.40) | nd | nd | nd |
| I-3-glc | 29.16 | 477;315 | 85.77 (0–171.53) | 5.28 (0–21.23) | 4.07 (0–12.22) | 11.34 (0–33.31) | nd | nd |
| I-3-rha | 30.24 | 461;315 | nd | 1.19 (0–10.68) | nd | nd | nd | 13.08 (0–26.16) |
| S-3-glc | 30.45 | 507;345 | 66.60 (63.35–69.85) | 13.58 (0–63.58) | nd | 11.25 (0–27.28) | 5.32 (0–15.97) | nd |
| Dq-3-acglc | 31.07 | 627;465,303 | nd | nd | nd | 5.89 (0–23.57) | 14.94 (0–44.81) | nd |
| K-3-rut | 36.88 | 539;285 | nd | 1.10 (0–9.88) | 58.62 (0–175.86) | 138.93 (0–475.01) | 33.51 (0–100.54) | nd |
| Flavan-3-ols (μg CE/g DW) | ||||||||
| Gallocatechin | 0.91 | 305;179,137 | 4.82 (0–9.64) | nd | nd | nd | nd | 18.03 (15.34–20.72) |
| Epigallocatechin | 2.33 | 305;179,141 | nd | nd | nd | nd | nd | 27.25 (21.05–33.45) |
| Catechin | 2.87 | 289 | 22.01 (9.90–34.13) | 2.49 (0–22.42) | nd | 6.42 (0–25.68) | 13.92 (0–25.90) | 32.85 (0–65.71) |
| Epicatechin | 6.44 | 289 | 6.54 (0–13.09) | 8.39 (0–28.66) | nd | nd | nd | 223.78 (163.53–284.03) |
| Procyanidin dimmer 1 | 2.18 | 577;425,289 | 62.05 (50.99–73.11) | 44.52 (0–381.80) | 187.58 (0–562.73) | 342.42 (6.45–1198.00) | 70.26 (0–154.99) | 72.41 (31.55–113.28) |
| Procyanidin dimmer 2 | 5.21 | 577;425,289 | 6.06 (0–12.12) | nd | nd | nd | nd | 26.72 (0–53.44) |
| Procyanidin dimmer 3 | 10.98 | 577;425,289 | 29.11 (0–58.22) | nd | nd | nd | nd | nd |
| Procyanidin trimer | 4.16 | 865; 577,289 | 4.77 (0–9.53) | nd | nd | 11.42 (0–45.67) | nd | nd |
| Compounds | Rt (min) | MS; MS2 (m/z) | Eu-grapes | As-grapes | Eu-As hybrids | Am-grapes | Eu-Am hybrids | Mu-grapes |
|---|---|---|---|---|---|---|---|---|
| Cinnamic acids (μg CAE/g DW) | ||||||||
| Chlorgenic acid | 0.56 | 191 | 0.67 (0–1.33) | 7.57 (0–10.16) | 5.83 (0–9.82) | 6.13 (0.38–8.85) | 4.86 (0.71–7.09) | 8.07 (7.89–8.26) |
| Caffeic acid | 1.19 | 179 | nd | 1.15 (0–10.36) | nd | 1.63 (0–5.55) | nd | nd |
| Caftaric acid | 1.30 | 311;179 | nd | 8.56 (0–24.16) | nd | nd | nd | nd |
| p-Coumaric acid | 2.90 | 163 | nd | 1.68 (0–11.45) | 3.28 (0–9.85) | 2.25 (0–8.99) | 7.92 (0–23.75) | 7.40 (0–14.80) |
| Ferulic acid | 3.01 | 193 | nd | 15.36 (0–58.44) | 17.81 (0–36.13) | 12.40 (0–32.58) | 5.13 (0–7.87) | nd |
| HE of caffeic acid | 5.63 | 341;179 | nd | nd | nd | nd | nd | 17.64 (0–35.29) |
| HE of p-coumaric acid | 6.11 | 325;163 | nd | 1.82 (0–16.36) | nd | nd | 3.26 (0–9.79) | 4.15 (0–8.29) |
| HE of ferulic acid | 7.47 | 355;193 | 0 (0-tr) | 16.98 (0–131.33) | 69.31 (10.26–183.29) | 13.18 (6.64–26.55) | 4.15 (1.25–8.97) | 4.17 (0–8.34) |
| Fertaric acid | 8.45 | 325;193 | nd | 58.15 (0–196.63) | 11.87 (0–25.11) | 8.50 (0–27.86) | 7.21 (0–18.15) | nd |
| Benzoic acids (μg GAE/g DW) | ||||||||
| HE of protocatechuic acid | 0.86 | 315;153 | 0.46 (0–0.91) | 6.96 (3.10–12.59) | 4.84 (tr-11.51) | 0.05 (0–0.19) | 4.38 (0–8.08) | 9.20 (7.07–11.34) |
| protocatechuic acid | 1.41 | 153 | nd | 0.17 (0–1.56) | nd | nd | nd | nd |
| p-Hydroxybenzoic acid | 1.77 | 137 | nd | 0.79 (0–3.70) | nd | nd | nd | nd |
| Ethyl gallate | 4.41 | 197;169 | nd | 1.45 (0–13.05) | nd | 0.94 (0–3.78) | 3.55 (0–6.08) | nd |
| HE of vanillic acid | 5.15 | 329;167 | 8.95 (7.73–10.17) | 3.99 (0–15.27) | 7.63 (4.39–11.17) | 6.75 (0.58–12.73) | 3.70 (tr-6.30) | 8.36 (6.96–9.75) |
| Ellagic acids (μg EAE/g DW) | ||||||||
| Ellagic acid-rha | 9.33 | 331;169,125 | nd | nd | nd | nd | nd | 16.49 (0–32.97) |
| HHDP-galloylglucose | 0.64 | 633;481,301 | nd | nd | nd | nd | nd | 140.16 (95.42–184.91) |
| HHDP-glucose | 3.81 | 481;421,301 | nd | nd | nd | nd | nd | 5.41 (0.00–10.82) |
| Ellagitannin 1 | 9.76 | 813;781,301 | nd | nd | nd | nd | nd | 92.91 (92.37–93.45) |
| Ellagitannin 2 | 14.47 | 831;813,301 | nd | nd | nd | nd | nd | 331.45 (303.32–359.59) |
| Stilbenes (μg RE/g DW) | ||||||||
| trans-Piceid | 10.19 | 389;227 | 2.50 (tr–5.01) | 0.96 (0–8.67) | 1.21 (0–3.64) | nd | nd | 28.21 (tr-56.41) |
| trans-Resveratrol | 23.67 | 227 | 94.44 (26.83–162.05) | 10.25 (0–67.09) | 9.10 (tr-27.31) | 0 (0-tr) | 0 (0-tr) | 13.71 (12.15–15.26) |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, L.; Zhang, Y.; Lu, J. Phenolic Contents and Compositions in Skins of Red Wine Grape Cultivars among Various Genetic Backgrounds and Originations. Int. J. Mol. Sci. 2012, 13, 3492-3510. https://doi.org/10.3390/ijms13033492
Zhu L, Zhang Y, Lu J. Phenolic Contents and Compositions in Skins of Red Wine Grape Cultivars among Various Genetic Backgrounds and Originations. International Journal of Molecular Sciences. 2012; 13(3):3492-3510. https://doi.org/10.3390/ijms13033492
Chicago/Turabian StyleZhu, Lei, Yali Zhang, and Jiang Lu. 2012. "Phenolic Contents and Compositions in Skins of Red Wine Grape Cultivars among Various Genetic Backgrounds and Originations" International Journal of Molecular Sciences 13, no. 3: 3492-3510. https://doi.org/10.3390/ijms13033492




