Chemical Reaction of Soybean Flavonoids with DNA: A Computational Study Using the Implicit Solvent Model
Abstract
:1. Introduction
2. Results and Discussion
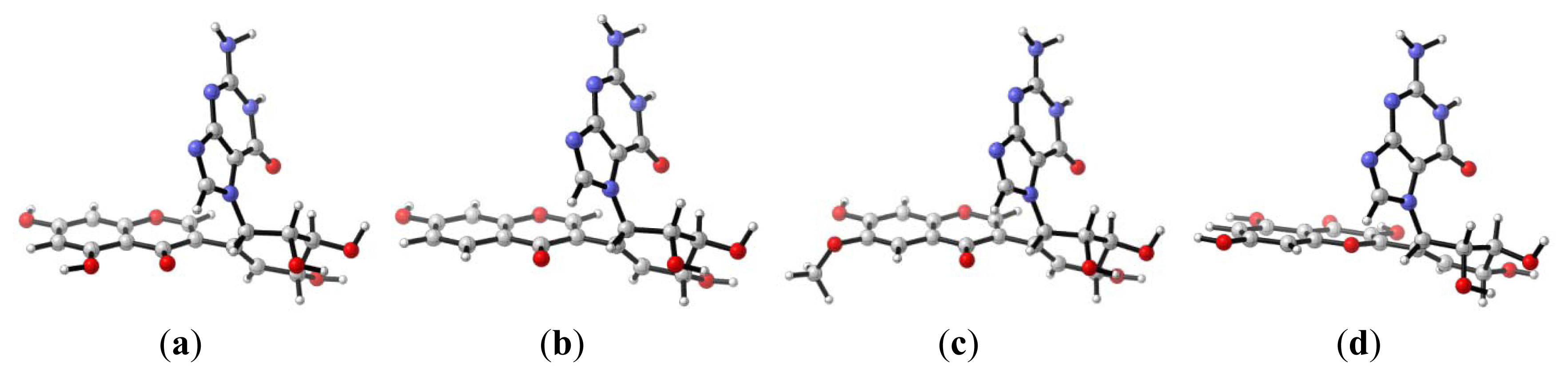
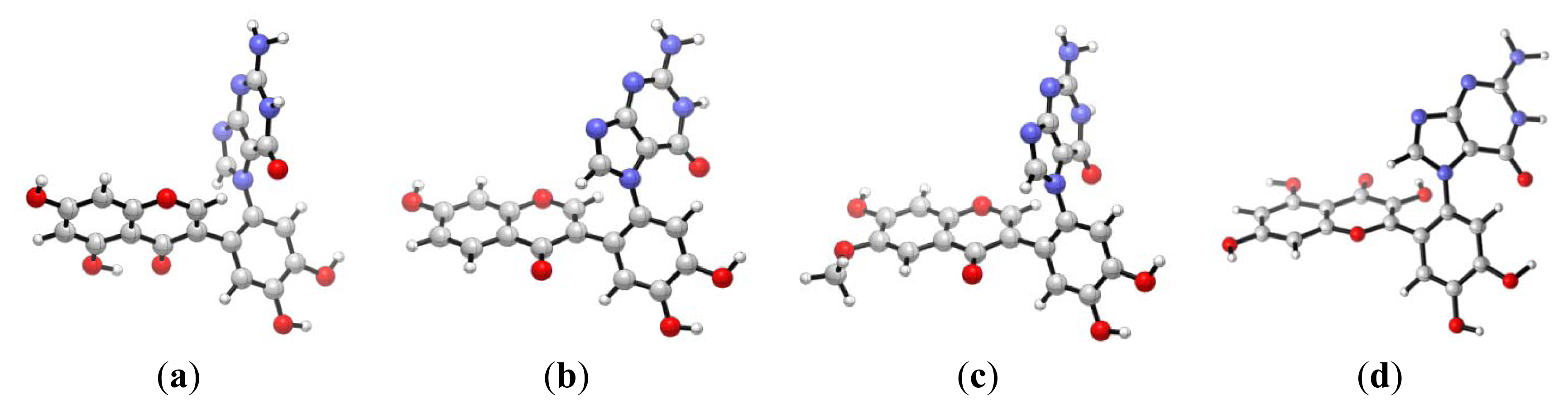
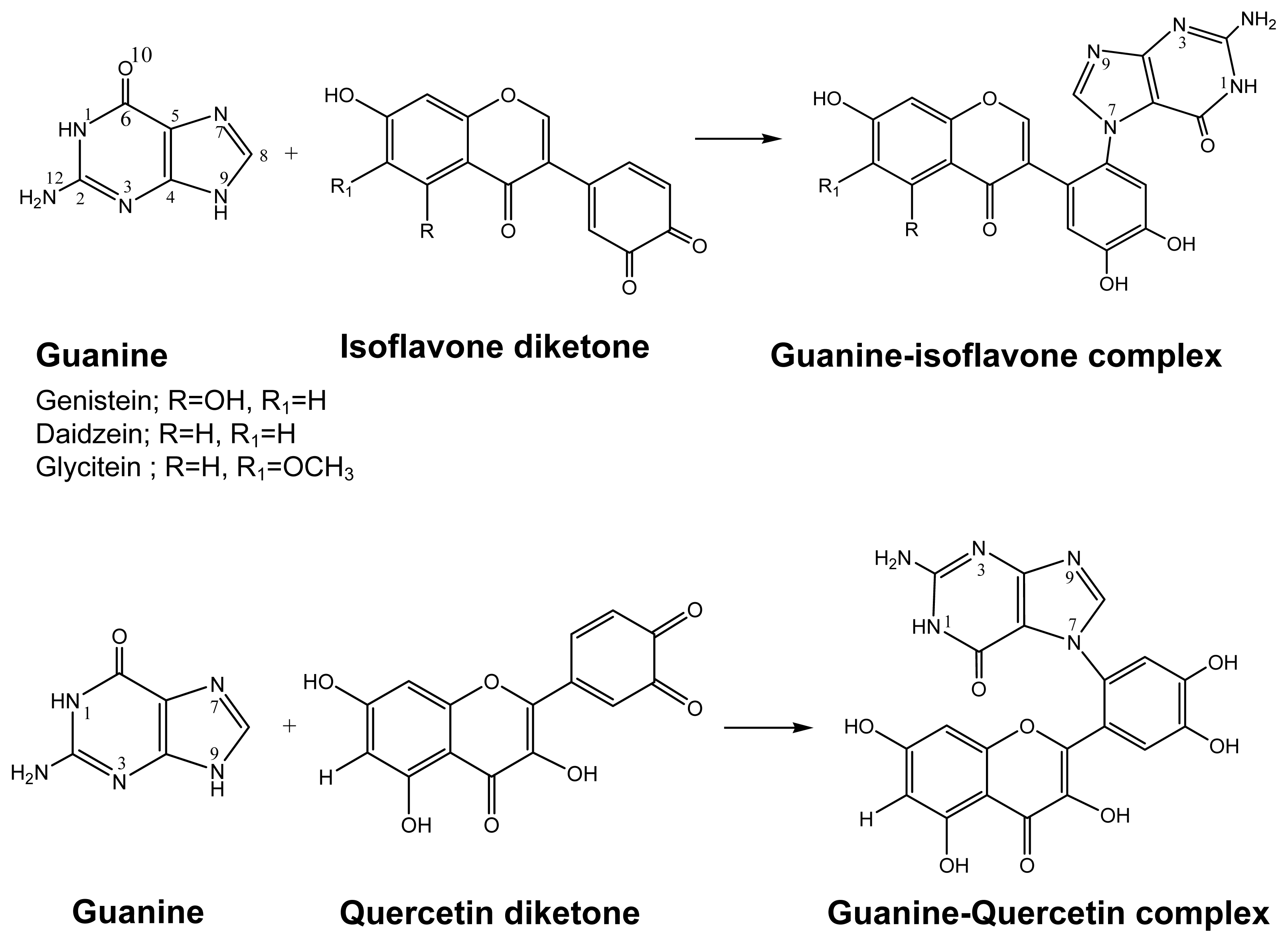
2.1. Diol Epoxide Mechanism
2.2. Diketone Mechanism
3. Computational Detail
4. Conclusions
Acknowledgments
References
- Underwood, J.C.E. General and Systematic Pathology, 3rd ed; Churchill Livingstone: Edinburgh, UK, 2000; pp. 374–375. [Google Scholar]
- Brookes, P.; Lawley, P.D. Evidence for the binding of polynuclear aromatic hydrocarbons to the nucleic acids of mouse skin: Relation between carcinogenic power of hydrocarbons and their binding to deoxyribonucleic acid. Nature 1964, 202, 781–784. [Google Scholar]
- Volk, D.E.; Rice, J.S.; Luxon, B.A.; Yeh, H.J.C.; Liang, C.; Xie, G.; Sayer, J.M.; Jerina, D.M.; Gorenstein, D.G. NMR evidence for syn-anti interconversion of a trans opened (10R)-dA adduct of benzo[a]pyrene (7S,8R)-diol (9R,10S)-epoxide in a DNA duplex. Biochemistry 2000, 39, 14040–14053. [Google Scholar]
- Smela, M.E.; Hamm, M.L.; Henderson, P.T.; Harris, C.M.; Harris, T.M.; Essigmann, J.M. The aflatoxin B1 formamidopyrimidine adduct plays a major role in causing the types of mutations observed in human hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2002, 99, 6655–6660. [Google Scholar]
- Stack, D.E.; Byun, J.; Gross, M.L.; Rogan, E.G.; Cavalieri, E.L. Molecular characteristics of catechol estrogen quinones in reactions with deoxyribonucleosides. Chem. Res. Toxicol 1996, 9, 851–859. [Google Scholar]
- Huetz, P.; Kamarulzaman, E.E.; Wahab, H.A.; Mavri, J. Chemical reactivity as a tool to study carcinogenicity: Reaction between estradiol and estrone 3,4-quinones ultimate carcinogens and guanine. J. Chem. Inf. Comput. Sci 2004, 44, 310–314. [Google Scholar]
- Watanabe, S.; Kobayashi, Y. Exogenous hormones and human cancer. Jpn. J. Clin. Oncol 1993, 23, 1–13. [Google Scholar]
- Cavalieri, E.; Rogan, E. Mechanisms of Tumor Initiation by Polycyclic Aromatic Hydrocarbons in Mammals. In The Handbook of Environmental Chemistry, PAHs and Related Compounds; Neilson, A.H., Ed.; Springer-Verlag: Berlin, Germany, 1998; Volume 3, pp. 81–117. [Google Scholar]
- Fauci, A.S.; Braunwald, E.; Kasper, D.L.; Hauser, S.L.; Longo, D.L.; Jameson, J.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 17th ed; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Mellon, I. DNA Repair. In Molecular and Biochemical Toxicology; Smart, R.C., Hodgson, E., Eds.; Wiley: Hoboken, NJ, USA, 2008; pp. 493–535. [Google Scholar]
- Murphy, P.A.; Farmakalidis, E.; Johnson, L.D. Isoflavone content of soya-based laboratory animal diets. Food Chem. Toxicol 1982, 20, 315–317. [Google Scholar]
- Elattar, T.M.; Virji, A.S. The inhibitory effect of curcumin, genistein, quercetin and cisplatin on the growth of oral cancer cells in vitro. Anticancer Res 2000, 20, 1733–1738. [Google Scholar]
- Dihal, A.A.; Woutersen, R.A.; Ommen, B.V.; Rietjens, I.M.; Stierum, R.H. Modulatory effects of quercetin on proliferation and differentiation of the human colorectal cell line Caco-2. Cancer Lett 2006, 238, 248–259. [Google Scholar]
- Dunnick, J.K.; Hailey, J.R. Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Fundam. Appl. Toxicol 1992, 19, 423–431. [Google Scholar]
- Pamukcu, A.M.; Yalciner, S.; Hatcher, J.F.; Bryan, G.T. Quercetin, a rat intestinal and bladder carcinogen present in bracken fern (Pteridium aquilinum). Cancer Res 1980, 40, 3468–3472. [Google Scholar]
- Erturk, E.; Hatcher, J.F.; Nunoya, T.; Pamukcu, A.M.; Bryan, G.T. Hepatic tumors in Sprague-Dawley (SD) and Fischer 344 (F) female rats chronically exposed to quercetin (Q) or its glycoside rutin (R). Proc. Am. Assoc. Cancer Res 1984, 25, 25–95. [Google Scholar]
- Morino, K.; Matsukara, N.; Kawachi, T.; Ohgaki, H.; Sugimura, T.; Hirono, I. Carcinogenicity test of quercetin and rutin in golden hamsters by oral administration. Carcinogenesis 1982, 3, 93–97. [Google Scholar]
- Pereira, M.A.; Grubbs, C.J.; Barnes, L.H.; Li, H.; Olson, G.R.; Eto, I.; Juliana, M.; Whitaker, L.M.; Kelloff, G.J.; Steele, V.E.; Lubet, R.A. Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,12-dimethylbenz[a]anthracene-induced mammary cancer in rats. Carcinogenesis 1996, 17, 1305–1311. [Google Scholar]
- Ishikawa, M.; Oikawa, T.; Hosokawa, M.; Hamada, J.; Morikawa, K.; Kobayashi, H. Enhancing effect of quercetin on 3-methylcholanthrene carcinogenesis in C57Bl/6 mice. Neoplasma 1985, 32, 435–441. [Google Scholar]
- Lamartiniere, C.A.; Murrill, W.B.; Manzolillo, P.A.; Zhang, J.X.; Barnes, S.; Zhang, X.; Wei, H.; Brown, N.M. Genistein alters the ontogeny of mammary gland development and protects against chemically-induced mammary cancer in rats. Proc. Soc. Exp. Biol. Med 1998, 217, 358–364. [Google Scholar]
- Song, T.T.; Hendrich, S.; Murphy, P.A. Estrogenic activity of glycitein, a soy isoflavone. J. Agric. Food Chem 1999, 47, 1607–1610. [Google Scholar]
- Shelby, M.D.; Newbold, R.R.; Tully, D.B.; Chae, K.; Davis, V.L. Assessing environmental chemicals for estrogenicity using a combination of in vitro and in vivo assays. Environ. Health Perspect 1996, 104, 1296–1300. [Google Scholar]
- Kalaiselvan, V.; Kalaivani, M.; Vijayakumar, A.; Sureshkumar, K.; Venkateskumar, K. Current knowledge and future direction of research on soy isoflavones as a therapeutic agents. Pharmacogn. Rev 2010, 4, 111–117. [Google Scholar]
- Zhao, L.; Chen, Q.; Brinton, R.D. Neuroprotective and neurotrophic efficacy of phytoestrogens in cultured hippocampal neurons. Exp. Biol. Med 2002, 227, 509–519. [Google Scholar]
- Anthony, M.S.; Clarkson, T.B.; Williams, J.K. Effects of soy isoflavones on atherosclerosis: Potential mechanisms. Am. J. Clin. Nutr 1998, 68, 1390S–1393S. [Google Scholar]
- Carroll, K.K. Review of clinical studies on cholesterol-lowering response to soy protein. J. Am. Diet. Assoc 1991, 91, 820–827. [Google Scholar]
- Cavalieri, E.L.; Rogan, E.G. The approach to understanding aromatic hydrocarbon carcinogenesis. The central role of radical cations in metabolic activation. Pharmacol. Ther 1992, 55, 183–199. [Google Scholar]
- Chakravarti, D.; Felling, J.C.; Cavalieri, E.L.; Rogan, E.G. Relating aromatic hydrocarbon-induced DNA adducts and c-H-ras mutations in mouse skin papillomas: The role of apurinic sites. Proc. Natl. Acad. Sci. USA 1995, 92, 10422–10426. [Google Scholar]
- Gelboin, H.V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: Role and regulation of mixed-function oxidases and related enzymes. Physiol. Rev 1980, 60, 1107–1166. [Google Scholar]
- Hall, M.; Grover, P.L. Policyclic Aromatic Hydrocarbons: Metabolism, Activation and Tumor Initiation. In Chemical Carcinogenesis and Mutagenesis I; Cooper, C.S., Grover, P.L., Eds.; Springer-Verlag: Berlin, Germany, 1990; pp. 327–372. [Google Scholar]
- Cavalieri, E.L.; Stack, D.E.; Devanesan, P.D.; Todorovic, R.; Dwivedy, I.; Higginbotham, S.; Johansson, S.L.; Patil, K.D.; Gross, M.L.; Gooden, J.K.; et al. Molecular origin of cancer: Catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc. Natl. Acad. Sci. USA 1997, 94, 10937–10942. [Google Scholar]
- Pauwels, W.; Veulemans, H. Comparison of ethylene, propylene and styrene 7,8-oxide in vitro adduct formation on N-terminal valine in human haemoglobin and on N-7-guanine in human DNA. Mutat. Res 1998, 418, 21–33. [Google Scholar]
- Goldstein, J.A.; Faletto, M.B. Advances in mechanisms of activation and deactivation of environmental chemicals. Environ. Health Perspect 1993, 100, 169–176. [Google Scholar]
- Rodeiro, I.; Donato, M.T.; Lahoz, A.; Garrido, G.; Delgado, R.; Gomez-Lechon, M.J. Interactions of polyphenols with the P450 system: Possible implications on human therapeutics. Mini Rev. Med. Chem 2008, 8, 97–106. [Google Scholar]
- Pelkonen, O.; Nebert, D.W. Metabolism of polycyclic aromatic hydrocarbons: Etiologic role in carcinogenesis. Pharmacol. Rev 1982, 34, 189–222. [Google Scholar]
- Dipple, A.; Moschel, R.C.; Bigger, C.A.H. Polynuclear aromatic carcinogens. Chem. Carcinog 1984, 1, 41–163. [Google Scholar]
- Russo, J.; Hu, Y.F.; Tahin, Q.; Mihaila, D.; Slater, C.; Lareef, M.H.; Russo, I.H. Carcinogenicity of estrogens in human breast epithelial cells. Acta Pathol. Microbiol. Immunol. Scand 2001, 109, 39–52. [Google Scholar]
- Galati, G.; Chan, T.; Wu, B.; O’Brien, P.J. Glutathione-dependent generation of reactive oxygen species by the peroxidase-catalyzed redox cycling of flavonoids. Chem. Res. Toxicol 1999, 12, 521–525. [Google Scholar]
- Awad, H.M.; Boersma, M.G.; Boeren, S.; van Bladeren, P.J.; Vervoort, J.; Rietjens, I.M.C.M. Structure—Activity study on the quinone/quinone methide chemistry of flavonoids. Chem. Res. Toxicol 2001, 14, 398–408. [Google Scholar]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D.; Schultz, R.A.; Ellenberger, T. DNA Repair and Mutagenesis, 2nd ed; American Society for Microbiology: Washington, DC, USA, 1995. [Google Scholar]
- Lagerqvist, A.; Håkansson, D.; Frank, H.; Seidel, A.; Jenssen, D. Structural requirements for mutation formation from polycyclic aromatic hydrocarbon dihydrodiol epoxides in their interaction with food chemopreventive compounds. Food Chem. Toxicol 2011, 49, 879–886. [Google Scholar]
- MacGregor, J.T.; Jurd, L. Mutagenicity of plant flavonoids: Structural requirements for mutagenic activity in Salmonella typhimurium. Mutat. Res 1978, 54, 297–309. [Google Scholar]
- Nagao, M.; Morita, N.; Yahagi, T.; Shimizu, M.; Kuroyanagi, M.; Fukuoka, M.; Yoshihira, K.; Natori, S.; Fujino, T.; Sugimura, T. Mutagenicities of 61 flavonoids and 11 related-compounds. Environ. Mutagen 1981, 3, 401–419. [Google Scholar]
- Vanderhoeven, J.C.M.; Bruggeman, I.M.; Debets, F.M.H. Genotoxicity of quercetin in cultured mammalian-cells. Mutat. Res 1984, 136, 9–21. [Google Scholar]
- Rietjens, I.M.C.M.; Boersma, M.G.; van der Woude, H.; Jeurissen, S.M.F.; Schutte, M.E.; Alink, G.M. Flavonoids and alkenylbenzenes: Mechanisms of mutagenic action and carcinogenic risk. Mutat. Res 2005, 574, 124–138. [Google Scholar]
- Stoewsand, G.S.; Anderson, J.L.; Boyd, J.N. Quercetin: A mutagen, not a carcinogen, in Fischer rats. J. Toxicol. Environ. Health 1984, 14, 105–114. [Google Scholar]
- Okamoto, T. Safety of quercetin for clinical application (Review). Int. J. Mol. Med 2005, 16, 275–278. [Google Scholar]
- Pamukcu, A. M.; Yalcener, S.; Hatcher, J. F. Quercetin as an intestinal and bladder carcinogen present in bracken fern. Teratog. Carcinog. Mutagen 1980, 1, 213–221. [Google Scholar]
- Pamukcu, A.M.; Yalciner, S.; Hatcher, J.F.; Bryan, G.T. Quercetin, a rat intestinal and bladder carcinogen present in bracken fern (Pteridium aquilinum). Cancer Res 1980, 40, 3468–3472. [Google Scholar]
- Win, W.; Cao, Z.; Peng, X.; Trush, M.A.; Li, Y. Different effects of genistein and resveratrol on oxidative DNA damage in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2002, 513, 113–120. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys 1992, 96, 2115–2160. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. II. The effect of the Perdew-Wang generalized-gradient correlation correction. J. Chem. Phys 1992, 97, 9173–9177. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys 1993, 98, 5648–5652. [Google Scholar]
- Yang, W.; Parr, R.G.; Lee, C. Various functionals for the kinetic energy density of an atom or molecule. Phys. Rev. A 1986, 34, 4586–4590. [Google Scholar]
- Tomasi, J.; Persico, M. Molecular interactions in solution: An overview of methods based on continuous distributions of the solvent. Chem. Rev 1994, 94, 2027–2094. [Google Scholar]
- Florián, J.; Warshel, A. Calculations of hydration entropies of hydrophobic, polar, and ionic solutes in the framework of the langevin dipoles solvation model. J. Phys. Chem. B 1999, 103, 10282–10288. [Google Scholar]
- Florián, J.; Warshel, A. Langevin dipoles model for ab initio calculations of chemical processes in solution: Parametrization and application to hydration free energies of neutral and ionic solutes and conformational analysis in aqueous solution. J. Phys. Chem. B 1997, 101, 5583–5595. [Google Scholar]
- Warhsell, A. Computer Modeling of Chemical Reactions in Enzymes and Solutions; Wiley-Interscience: New York, NY, USA, 1997. [Google Scholar]
- Wattenberg, L.W. Chemoprevention of cancer. Cancer Res 1985, 45, 1–8. [Google Scholar]
- Murray, G.I.; Taylor, V.E.; McKay, J.A.; Weaver, R.J.; Ewen, S.W.B.; Melvin, W.T.; Burke, M.D. The immunohistochemical localization of drug-metabolizing enzymes in prostate cancer. J. Pathol 1995, 177, 147–152. [Google Scholar]
- Murray, G.I.; Taylor, V.E.; McFadyen, M.C.; McKay, J.A.; Greenlee, W.F.; Burke, M.D.; Melvin, W.T. Tumor-speci.c expression of cytochrome P450 CYP1B1. Cancer Res 1997, 57, 3026–3031. [Google Scholar]
- Shimada, T.; Fujii-Kuriyama, Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci 2004, 95, 1–6. [Google Scholar]
- Dunning, A.M.; Healey, C.S.; Pharoah, P.D.P.; Teare, M.D.; Ponder, B.A.J.; Easton, D.F. A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol. Biomark. Prev 1999, 8, 843–854. [Google Scholar]
- Kržan, M.; Mavri, J. Carcinogenicity of styrene oxide: Calculation of chemical reactivity. Croat. Chem. Acta 2009, 82, 317–322. [Google Scholar]
- Matsui, A.; Ikeda, T.; Enomoto, K.; Nakashima, H.; Omae, K.; Watanabe, M.; Hibi, T.; Kitajima, M. Progression of human breast cancers to the metastatic state is linked to genotypes of catechol-O-methyltransferase. Cancer Lett 2000, 150, 23–31. [Google Scholar]
- Yager, J.D.; Liehr, J.G. Molecular mechanisms of estrogen carcinogenesis. Annu. Rev. Pharmacol. Toxicol 1996, 36, 203–232. [Google Scholar]
- Wang, M.Y.; Liehr, J.G. Identification of fatty acid hydroperoxide cofactors in the cytochrome P450-mediated oxidation of estrogens to quinone metabolites. Role and balance of lipid peroxides during estrogen-induced carcinogenesis. J. Biol. Chem 1994, 269, 284–291. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.02; Gaussian, Inc: Wallingford, CT, USA, 2008. [Google Scholar]

| DFT Method (B3LYP/6-31G(d)) | ||||||
|---|---|---|---|---|---|---|
| ΔE a | ΔZPE b | PCM method | LD method | |||
| ΔGhydr c | ΔGreact d | ΔGhydr | ΔGreact | |||
| Diol epoxide mechanism | ||||||
| Genistein | −30.04 | 2.19 | 1.34 | −26.51 | 6.50 | −21.35 |
| Daidzein | −29.74 | 2.21 | 1.20 | −26.33 | 5.80 | −21.73 |
| Glycitein | −29.66 | 2.24 | 1.85 | −25.57 | 5.90 | −21.52 |
| Quercetin | −31.95 | 2.55 | 1.52 | −27.88 | 11.10 | −18.30 |
| Diketone mechanism | ||||||
| Genistein | −26.83 | 3.73 | 11.31 | −11.79 | 5.82 | −17.28 |
| Daidzein | −22.79 | 3.50 | 7.85 | −11.44 | 3.28 | −16.01 |
| Glycitein | −22.94 | 3.50 | 7.65 | −11.79 | 1.32 | −18.12 |
| Quercetin | −20.44 | 1.84 | 0.42 | −18.18 | 12.40 | −6.20 |
| HF Method (HF/6-31G(d)) | ||||||
| ΔE a | ΔZPE b | PCM method | LD method | |||
| ΔGhydr c | ΔGreact d | ΔGhydr | ΔGreact | |||
| Diol epoxide mechanism | ||||||
| Genistein | −31.68 | 2.43 | 1.48 | −27.77 | 18.60 | −10.65 |
| Daidzein | −31.26 | 2.40 | 1.54 | −27.32 | 17.00 | −11.86 |
| Glycitein | −31.16 | 2.40 | 1.85 | −26.91 | 15.10 | −13.66 |
| Quercetin | −32.79 | 2.58 | 2.23 | −27.98 | 13.90 | −16.31 |
| Diketone mechanism | ||||||
| Genistein | −23.30 | 1.57 | 1.39 | −20.34 | 23.30 | 1.57 |
| Daidzein | −17.58 | 1.59 | 0.93 | −15.06 | 17.70 | 1.71 |
| Glycitein | −17.49 | 1.60 | 1.06 | −14.83 | 21.70 | 5.81 |
| Quercetin | −14.45 | 1.52 | 1.06 | −11.87 | 14.50 | 1.57 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abdallah, H.H.; Mavri, J.; Repič, M.; Lee, V.S.; Wahab, H.A. Chemical Reaction of Soybean Flavonoids with DNA: A Computational Study Using the Implicit Solvent Model. Int. J. Mol. Sci. 2012, 13, 1269-1283. https://doi.org/10.3390/ijms13021269
Abdallah HH, Mavri J, Repič M, Lee VS, Wahab HA. Chemical Reaction of Soybean Flavonoids with DNA: A Computational Study Using the Implicit Solvent Model. International Journal of Molecular Sciences. 2012; 13(2):1269-1283. https://doi.org/10.3390/ijms13021269
Chicago/Turabian StyleAbdallah, Hassan H., Janez Mavri, Matej Repič, Vannajan Sanghiran Lee, and Habibah A. Wahab. 2012. "Chemical Reaction of Soybean Flavonoids with DNA: A Computational Study Using the Implicit Solvent Model" International Journal of Molecular Sciences 13, no. 2: 1269-1283. https://doi.org/10.3390/ijms13021269




