Imidazolines as Non-Classical Bioisosteres of N-Acyl homoserine lactones and Quorum Sensing Inhibitors
Abstract
:1. Introduction
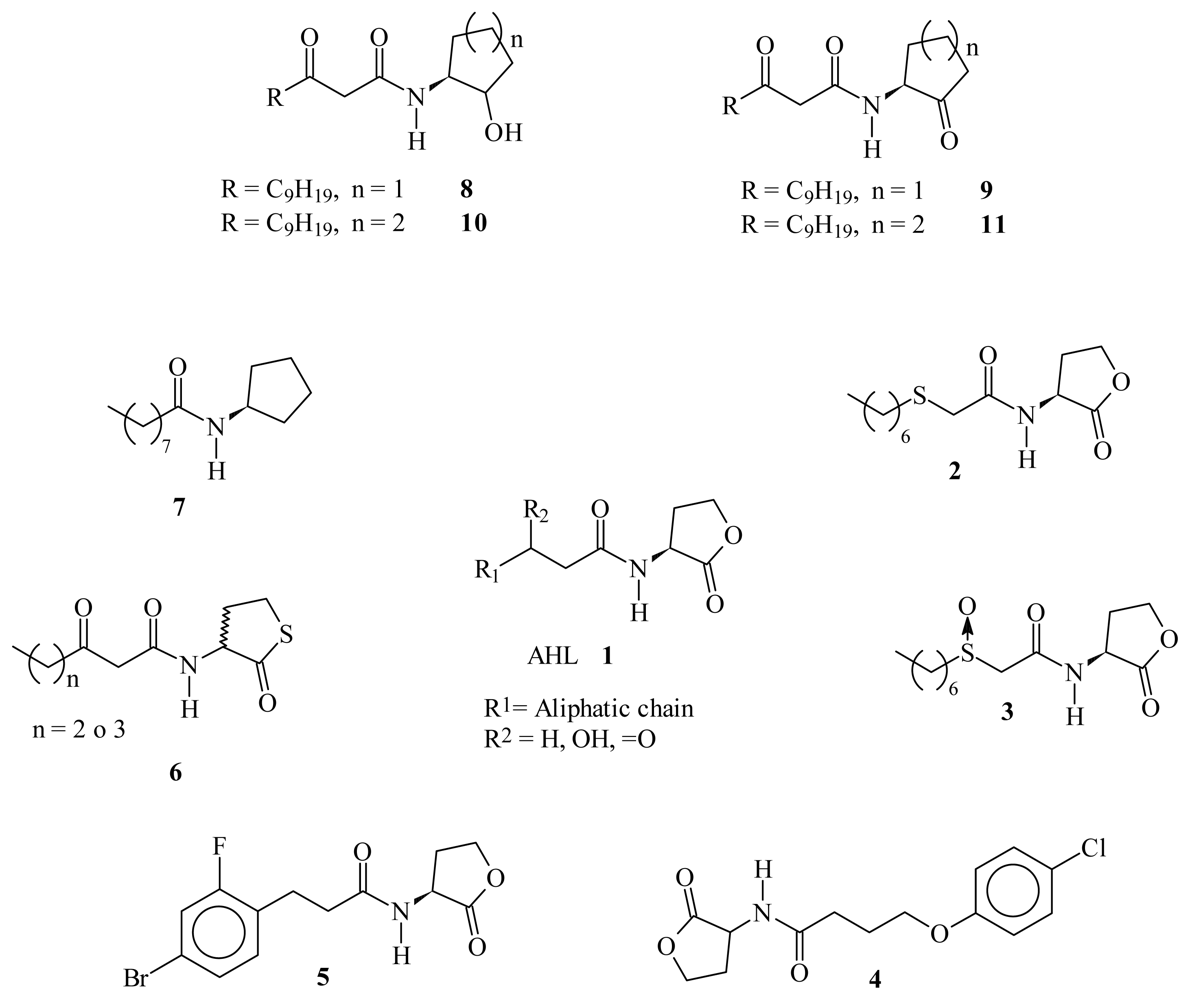
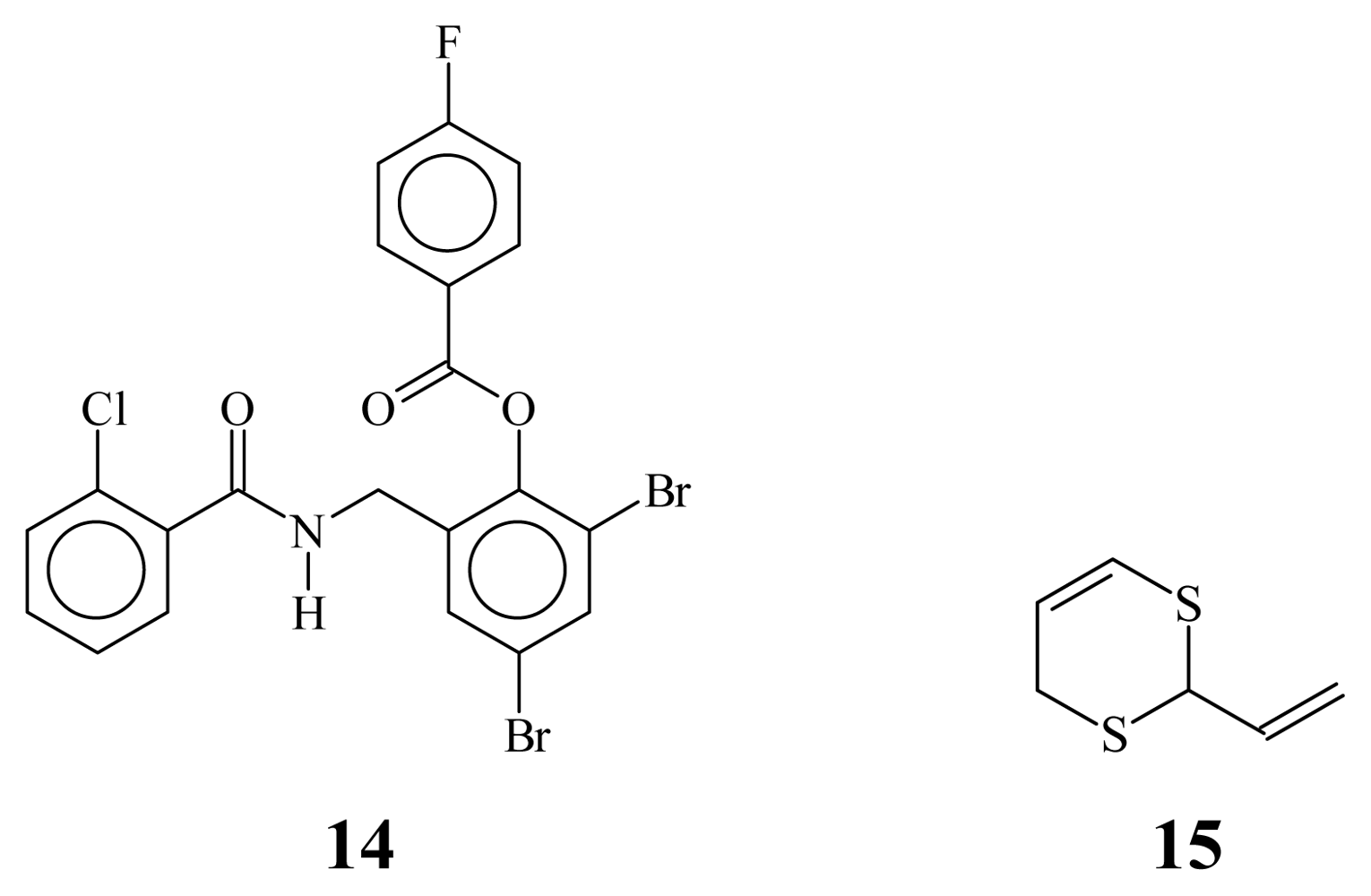
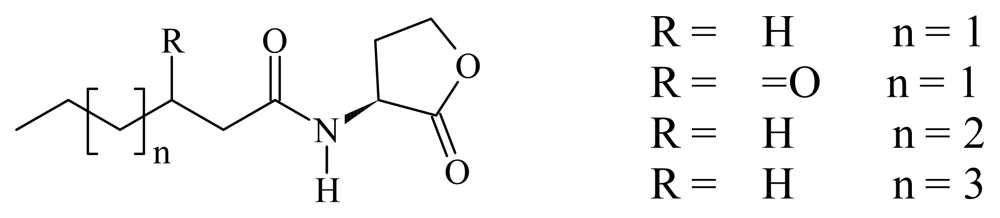
2. Results and Discussion
2.1. Bioisosteric Design
2.2. Chemistry
2.3. Biology
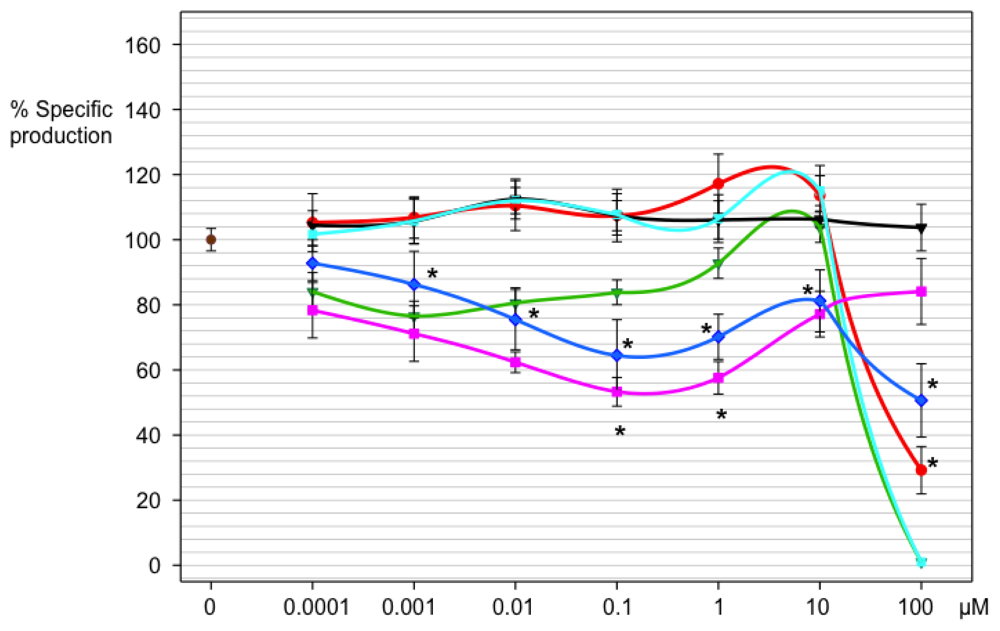
2.3.1. Evaluation of the Imidazoline Derivatives 18a–18f on Chromobacterium violaceum wt
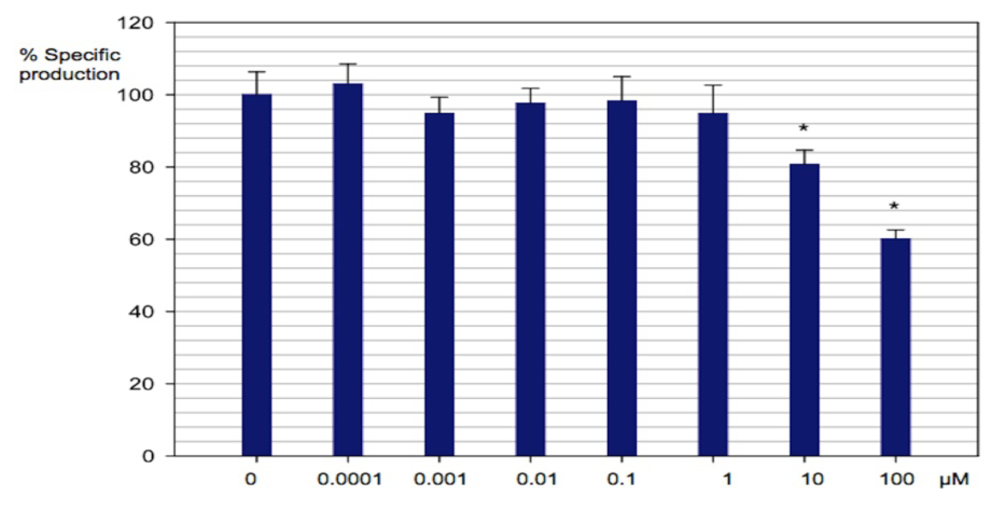
2.3.2. Effect of Compounds 18a–18f on the Viability of Chromobacterium violaceum Wild Type
2.3.3. Effects of the Imidazoline Derivatives on Chromobacterium violaceum
2.3.4. Evaluation of N-[4-Phenyl-(imidazo-2-yl)]-nonamide on Serratia marcescens ATCC 8100
3. Experimental Section
3.1. Experimental Chemical Section
3.1.1. General Procedure for the Synthesis of 4-Alkyloxybenzaldehydes [25]
3.1.2. General Procedure for the Synthesis of 3-Alkylbenzaldehydes
3.1.3 General Procedure for the Synthesis of Imidazolines from Benzaldehydes
3.1.4. Synthesis of N-4-Benzonitrile Alkylamides
3.1.5. Synthesis of N-[4-Phenyl-(imidazo-2-yl)]-alkylamides
3.2. Experimental Biological Section
3.2.1. Bacterial Strains
3.2.2. Evaluation of the Effect of Bioisosteres on Cell Viability
3.2.3. Quantification of Violacein in Chromobacterium violaceum Wild Type
3.2.4 Quantification of Prodigiosin on Serratia marcescens ATCC 8100
3.2.5. Extraction and Quantification of Prodigiosin
4. Conclusions
Acknowledgements
References and Notes
- Fleming, A. Penicillin. Presented at Nobel Lecture 1945, Sweden, 11 December 1945.
- Raffa, R.B.; Iannuzzo, J.R.; Levine, D.R.; Saeid, K.K.; Schwartz, R.C.; Sucic, N.T.; Terleckyj, O.D.; Young, J.M. Bacterial communication (“Quorum Sensing”) via ligands and receptors: A novel pharmacologic target for the design of antibiotic drugs. J. Pharmacol. Exp. Ther 2005, 312, 417–423, and references therein. [Google Scholar]
- Shih, P.-Ch.; Wang, Ch.-T. Effects of quorum-sensing deficiency on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. J. Antimicrob. Chemother. 2002, 49, 309–314. [Google Scholar]
- Otero Casal, A.M.; Munoz Crego, A.; Bernárdez Hermida, M.I.; Fábregas Casal, J. Quorum Sensing. El Lenguaje de las Bacterias (in Spanish); Editorial Acribia: Zaragoza, Espana, 2005. [Google Scholar]
- Galloway, W.R.; Hodgkinson, J.T.; Bowden, S.D.; Welch, M.; Spring, D.R. Quorum Sensing in gram-negative bacteria: Small-molecule modulation of AHL and AI-2 Quorum Sensing pathways. Chem. Rev 2011, 111, 28–67. [Google Scholar]
- Blackwell, H.E.; Fuqua, C. Introduction to bacterial signals and chemical communication. Chem. Rev 2011, 111, 1–3. [Google Scholar]
- Quorum sensing is a word which consists of a Latin word “quorum” and a English word “sensing” but his origin is Latin; in consequence we consider all the term must be written in italics.
- Moreira, L.L.; Barreiro, E.J. Bioisosterism: A useful strategy for molecular modification and drug design. Curr. Med. Chem 2005, 12, 23–49. [Google Scholar]
- Patani, A.G.; LaVoie, J.E. Bioisosterism. A rational approach in drug design. Chem. Rev 1996, 96, 3147–3176. [Google Scholar]
- Persson, T.; Hansen, T.H.; Rasmussen, T.B.; Skindersø, M.E.; Givskov, M.; Nielsen, J. Rational design and synthesis of new Quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org. Biomol. Chem 2005, 3, 253–262. [Google Scholar]
- Chen, G.; Swem, L.R.; Swem, D.L.; Stauff, D.L.; O’Loughlin, C.T.; Jeffrey, P.D.; Bassler, B.L.; Hughson, F.M. A strategy for antagonizing quorum sensing. Mol. Cell 2011, 42, 199–209. [Google Scholar]
- Geske, G.D.; Wezeman, R.J.; Siegel, A.P.; Blackwell, H.E. Small molecule inhibitors of bacterial Quorum Sensing and biofilm formation. J. Am. Chem. Soc 2005, 127, 12762–12763. [Google Scholar]
- Geske, G.D.; O’Neill, J.C.; Miller, D.M.; Mattmann, M.E.; Blackwell, H.E. Modulation of bacterial Quorum Sensing with synthetic ligands: Systematic evaluation of N-Acylated homoserine lactones in multiple species and new insights into their mechanisms of action. J. Am. Chem. Soc 2007, 129, 13613–13625. [Google Scholar]
- Janssens, J.C.A.; Steenackers, H.; Metzger, K.; Daniels, R.; Ptacek, D.; Verhoeven, T.; Hermans, K.; Vanderleyden, J.; de Vos, D.E.; de Keersmaecker, S.C.J. Interference with the Quorum Sensing systems of Salmonella enterica serovar typhimurium: Possibilities and implications. Commun. Agric. Appl. Biol. Sci 2007, 72, 35–39. [Google Scholar]
- Tomohiro, M.; Toshitaka, S.; Kiyomi, T.; Masashi, K.; Norihiro, K.; Junichi, K.; Tsukasa, I. Inhibition of quorum sensing in Serratia marcescens AS-1 by synthetic analogs of N-Acylhomoserine lactone. Appl. Environ. Microbiol 2007, 6339–6344. [Google Scholar]
- Smith, K.M.; Yigong, B.; Suga, H. Induction and inhibition of Pseudomonas aeruginosa Quorum Sensing by synthetic autoinducer analogs. Chem. Biol 2003, 10, 81–89. [Google Scholar]
- Janssens, J.C.A.; Metzger, K.; Daniels, R.; Ptacek, D.; Verhoeven, T.; Habel, L.W.; Vanderleyden, J.; de Vos, D.E.; de Keersmaecker, S.C. Synthesis of N-acyl homoserine lactone analogs reveals strong activators of SdiA, the salmonella enterica serovar typhimurium LuxR homolog. Appl. Environ. Microbiol 2007, 73, 535–544. [Google Scholar]
- Smith, K.M.; Bu, Y.; Suga, H. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem. Biol 2003, 10, 563–571. [Google Scholar]
- Yang, L.; Rybtke, M.T.; Jakobsen, T.H.; Hentzer, M.; Bjarnsholt, T.; Givskov, M.; Tolker-Nielsen, T. Computer-aided identification of recognized drugs as Pseudomonas aeruginosa Quorum Sensing inhibitors. Antimicrob. Agents Chemother 2009, 53, 2432–2443. [Google Scholar]
- Müh, U.; Schuster, M.; Heim, R.; Singh, A.; Olson, E.R.; Greenberg, P.E. Novel Pseudomonas aeruginosa Quorum-sensing inhibitors identified in an ultra-high-throughput screen. Antimicrob. Agents Chemother 2006, 50, 3674–3679. [Google Scholar]
- Zhu, H.; Sun, S.J. Inhibition of bacterial quorum sensing regulated behaviors by Tremella fuciformis extract. Curr. Microbiol 2008, 57, 418–422. [Google Scholar]
- Nagy, M.M. Quorum Sensing Inhibitory Activities of Various Folk-Medicinal Plants and the Thyme-tetracycline Effect. Biology Dissertations; Georgia State University, 2010. Paper 90. Available online: http://digitalarchive.gsu.edu/biology_diss/90 accessed on 11 January 2012.
- Mueh, U.; Hare, B.J.; Duerkop, B.A.; Schuster, M.; Hanzelka, B.L.; Heim, R.; Olson, E.R.; Greenberg, P.E. A structurally unrelated mimic of a Pseudomonas aeruginosa Acyl-homoserine lactone Quorum-sensing signal. Proc. Natl. Acad. Sci. USA 2006, 103, 16948–16952. [Google Scholar]
- Tomohiro, M.; Masashi, K.; Katsumasa, F.; Norihiro, K.; Tsukasa, I. N-Acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain ATCC 12472. FEMS Microbiol. Lett 2008, 279, 124–130. [Google Scholar]
- Cristóbal, D.G. Síntesis de N,N-bis-[4′(2-bromo etoxi)benciliden] etilendiamina y su posible aplicación como monómero de supramoléculas. In Tesis Profesional; ENCB-IPN: Mexico, 2007. [Google Scholar]
- Gelens, E.; Smeets, L.; Sliedregt, L.A.J.M.; van Steen, B.J.; Kruse, Ch.G.; Leurs, R.; Orru, R.V.A. An atom efficient and solvent-free synthesis of structurally diverse amides using microwave. Tetrahedron Lett. 2005, 46, 3751–3754. [Google Scholar]
- Partridge, M.W.; Turner, H.A. Derivatives of n-Hexyl phenyl ether as antituberculous compounds. J. Pharm. Pharmacol 1953, 5, 111–116. [Google Scholar]
- Harfenist, M.; Soroko, F.E.; McKenzie, G.M. 2-(Alkoxyaryl)-2-imidazoline monoamine oxidase inhibitors with antidepressant activity. J. Med. Chem 1978, 21, 405–409. [Google Scholar]
- Fujioka, H.; Murai, K.; Kubo, O.; Ohbay, Y.; Kita, Y. One-pot synthesis of imidazolines from aldehydes: Detailed study about solvents and substrates. Tetrahedron 2007, 63, 638–643. [Google Scholar]
- Pathan, M.Y.; Paike, V.V.; Pachmase, P.R.; More, S.P.; Ardhapure, S.S.; Pawar, R.P. Microwave-assisted facile Synthesis of 2-substituted 2-imidazolines. ARKIVOC 2006, 205–210. [Google Scholar]
- Pez, D.; Leal, I.; Zuccotto, F.; Boussard, C.; Brun, R.; Croft, S.L.; Yardley, V.; Ruiz, L.M.P.; Gonzalez, D.P.; Gilberta, I.H. 2,4-Diaminopyrimidines as inhibitors of leishmanial and trypanosomal dihydrofolate reductase. Bioorg. Med. Chem. 2003, 11, 4693–4711. [Google Scholar]
- Sing, B.; Pandey, A. Synthesis, characterization and mesomorphic properties of unsymmetrical N-(O-hydroxybenzylidene)-N-(4-n-alkoxybenzylidene) azines and their copper(II) complexes. Mol. Cryst. Liq. Cryst 2010, 37, 57–67. [Google Scholar]
- Martinelli, D.; Grossmann, G.; Séquin, U.; Brandl, H.; Bachofen, R. Effects of natural and chemically synthesized furanones on Quorum Sensing in Chromobacterium violaceum. BMC Microbiol 2004, 4, 25. [Google Scholar]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum Sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acyl homoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar]
- Mall, T.; Follath, F.; Salfinger, M.; Ritz, R.; Reber, H. Moxalactam in nosocomial infections with Serratia marcescens. Intensive Care Med 1985, 11, 179–183. [Google Scholar]
- Horng, Y.-T.; Deng, S.-C.; Daykin, M.; Soo, P.-C.; Wei, J.-R.; Luh, K.-T.; Ho, S.-W.; Swift, S.; Lai, H.-C.; Williams, P. The LuxR family protein SpnR functions as a negative regulator of N-acylhomoserine lactone-dependent Quorum Sensing in Serratia marcescens. Mol. Microbiol 2002, 6, 1655–1671. [Google Scholar]

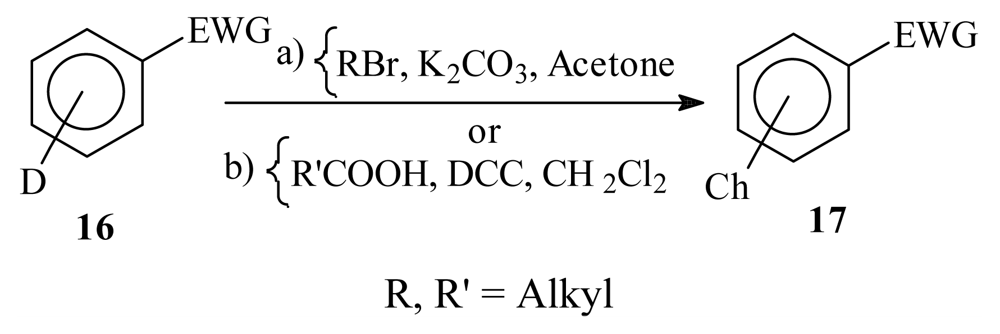 | |||||
|---|---|---|---|---|---|
| D | EWG | Reagents and reaction conditions | Ch | Compound number | Isolated yield (%) |
| p-OH | CHO | a, reflux | O-nC6H13 | 17a | 80 |
| p-OH | CHO | a, reflux | O-nC9H19 | 17b | 85 |
| m-OH | CHO | a, reflux | O-nC6H13 | 17c | 81 |
| m-OH | CHO | a, reflux | O-nC9H19 | 17d | 81 |
| NH2 | CN | b, r.t. | NHCO-nC5H11 | 17e | 70 |
| NH2 | CN | b, r.t. | NHCO-nC8H17 | 17f | 80 |
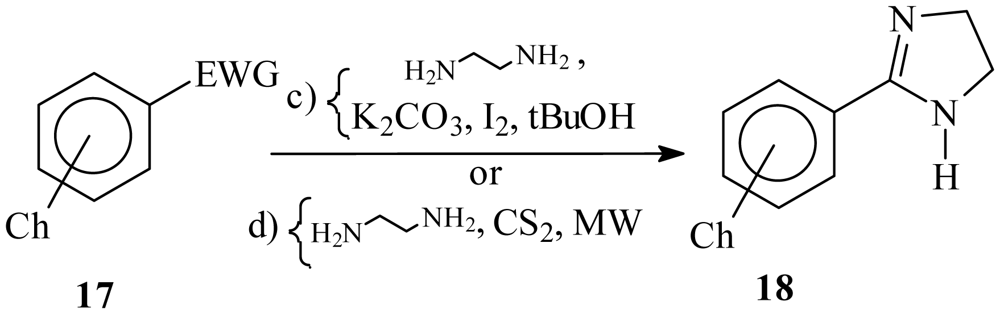 | ||||
|---|---|---|---|---|
| Ch | EWG | Reagents and conditions | Compound number | Isolated yield (%) |
| p-O-nC6H13 | CHO | c, reflux | 18a | Quantitative |
| p-O-nC9H19 | CHO | c, reflux | 18b | Quantitative |
| m-O-nC6H13 | CHO | c, reflux | 18c | Quantitative |
| m-O-nC9H19 | CHO | c, reflux | 18d | Quantitative |
| NHCO-nC5H11 | CN | d, MW | 18e | 50 |
| NHCO-nC8H17 | CN | d, MW | 18f | 60 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reyes-Arellano, A.; Bucio-Cano, A.; Montenegro-Sustaita, M.; Curiel-Quesada, E.; Salgado-Zamora, H. Imidazolines as Non-Classical Bioisosteres of N-Acyl homoserine lactones and Quorum Sensing Inhibitors. Int. J. Mol. Sci. 2012, 13, 1284-1299. https://doi.org/10.3390/ijms13021284
Reyes-Arellano A, Bucio-Cano A, Montenegro-Sustaita M, Curiel-Quesada E, Salgado-Zamora H. Imidazolines as Non-Classical Bioisosteres of N-Acyl homoserine lactones and Quorum Sensing Inhibitors. International Journal of Molecular Sciences. 2012; 13(2):1284-1299. https://doi.org/10.3390/ijms13021284
Chicago/Turabian StyleReyes-Arellano, Alicia, Alejandro Bucio-Cano, Mabel Montenegro-Sustaita, Everardo Curiel-Quesada, and Héctor Salgado-Zamora. 2012. "Imidazolines as Non-Classical Bioisosteres of N-Acyl homoserine lactones and Quorum Sensing Inhibitors" International Journal of Molecular Sciences 13, no. 2: 1284-1299. https://doi.org/10.3390/ijms13021284
APA StyleReyes-Arellano, A., Bucio-Cano, A., Montenegro-Sustaita, M., Curiel-Quesada, E., & Salgado-Zamora, H. (2012). Imidazolines as Non-Classical Bioisosteres of N-Acyl homoserine lactones and Quorum Sensing Inhibitors. International Journal of Molecular Sciences, 13(2), 1284-1299. https://doi.org/10.3390/ijms13021284




