Effects of Schizonepetin on Activity and mRNA Expression of Cytochrome P450 Enzymes in Rats
Abstract
:1. Introduction
2. Results and Discussion
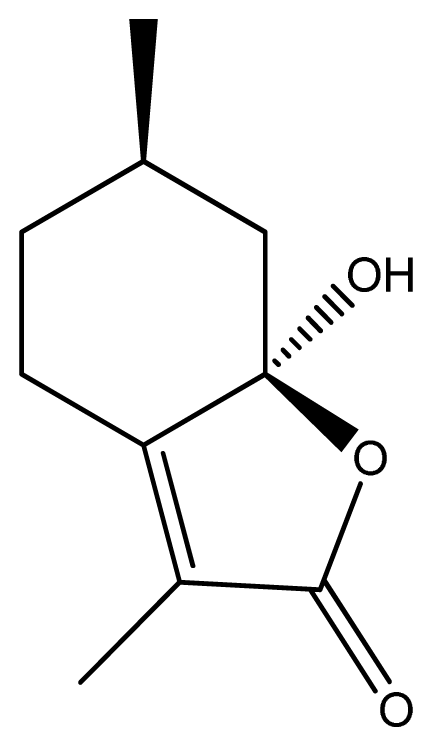
2.1. Effect of Schizonepetin on the Activities of CYP3A1/2 in Rats
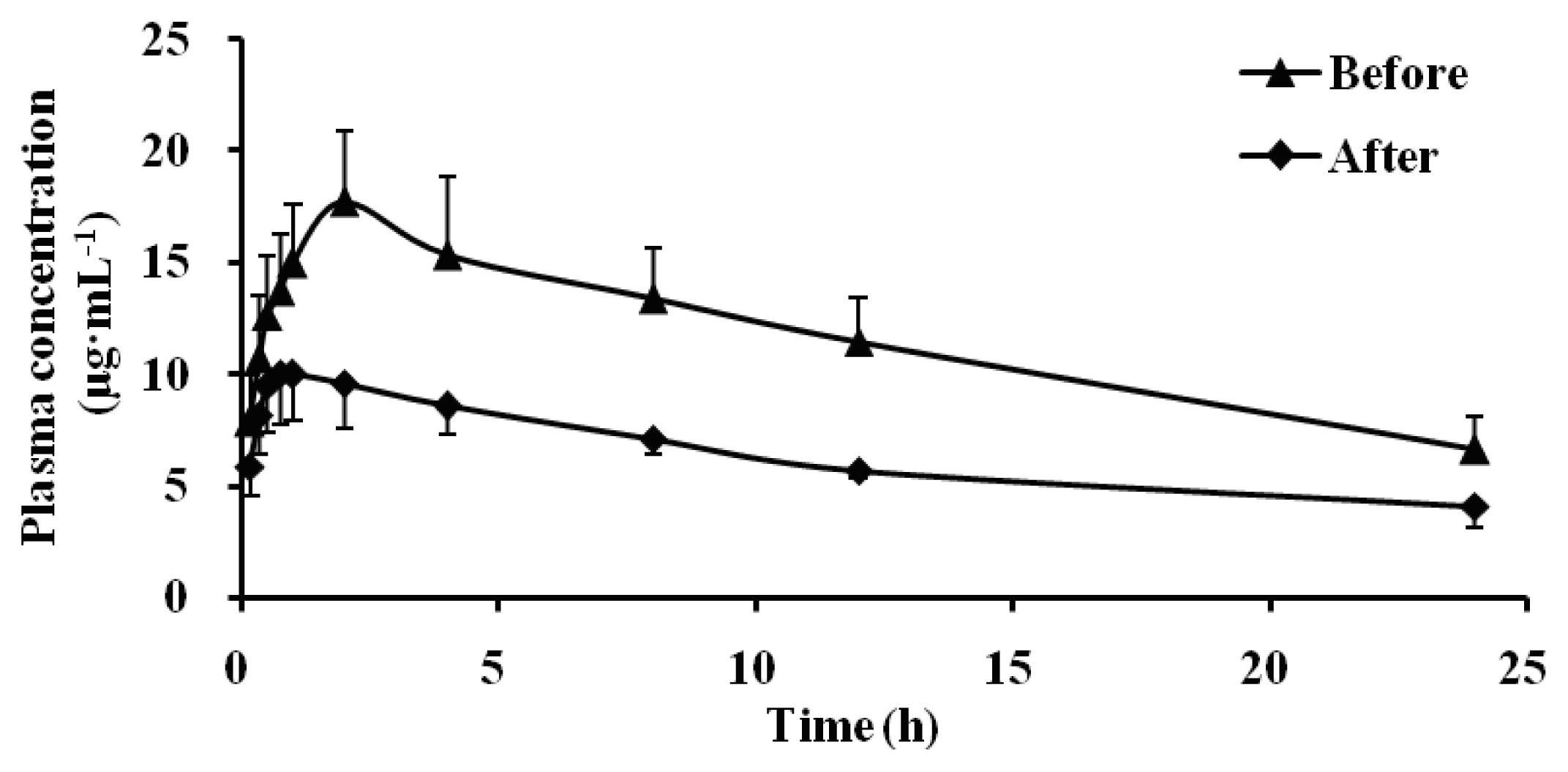
2.2. Effect of Schizonepetin on the Activities of CYP1A2 in Rats
2.3. Effect of Schizonepetin on the Activities of CYP2E1 in Rats
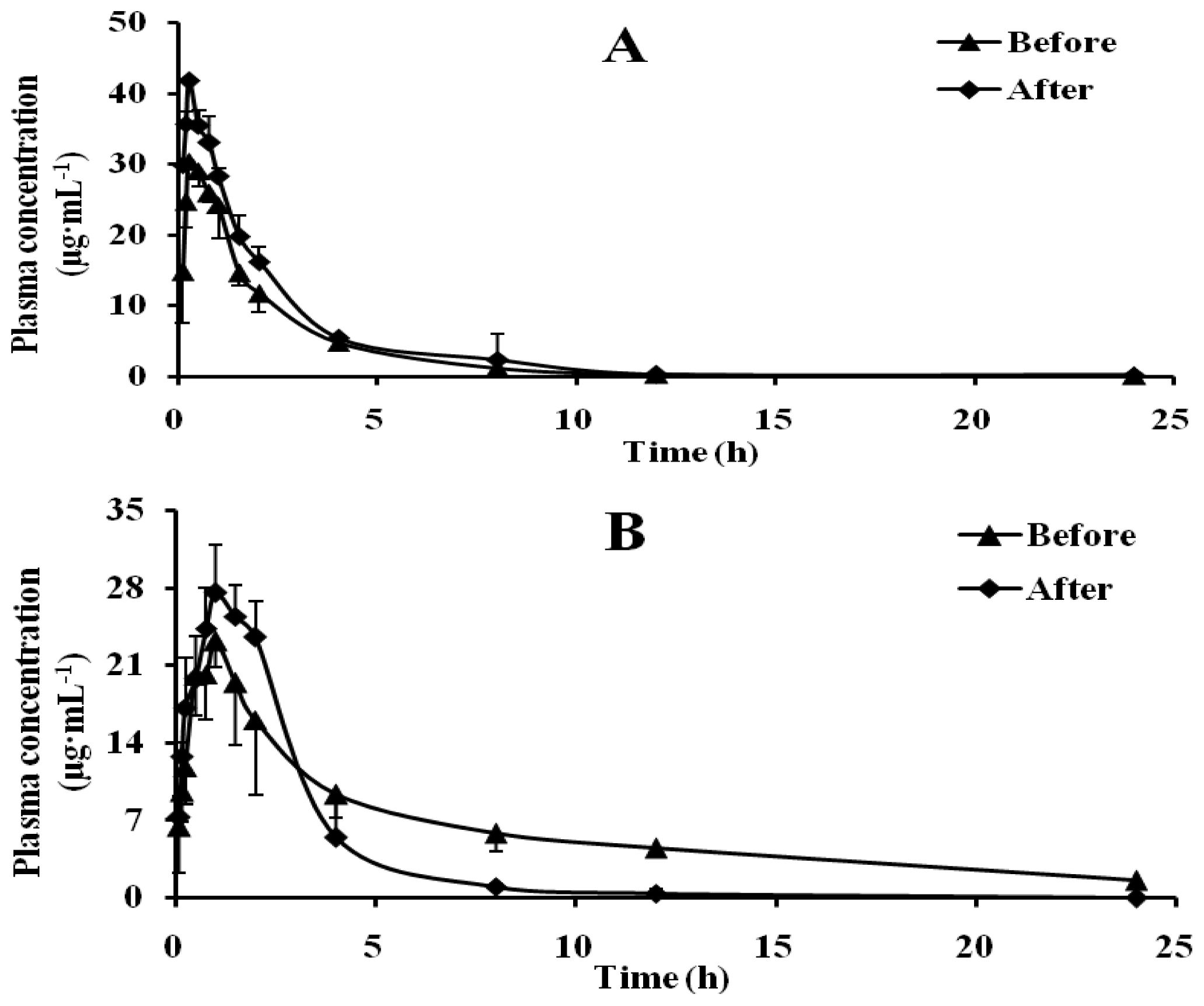
2.4. Effect of Schizonepetin on the Activities of CYP2C19 in Rats
2.5. Effect of Schizonepetin on the Activities of CYP2D6 in Rats
2.6. Effect of Schizonepetin on the Activities of CYP3A1, CYP1A2 and CYP2E1 mRNA Expression in Rats
2.7. Discussion
3. Experimental Section
3.1. Experimental Material and Instrument
3.1.1. Apparatus
3.1.2. Chemicals and Reagents
3.1.3. Animals
3.2. In Vivo Assay
3.2.1. Drug Administration and Sampling
3.2.2. Sample Extraction Procedure
3.2.3. Chromatographic Methods
3.2.4. Statistical Analysis
3.3. Method of Effects of Schizonepetin on mRNA Expression of Cytochrome P450 Enzymes in Rat
3.3.1. Drug Administration and Sampling
3.3.2. Total RNA Isolation
3.3.3. Synthesis of cDNA
3.3.4. Polymerase Chain Reaction
- GAPDH:
- Forward: 5′-CAAGGTCATCCATGACAACTTTG-3′
- Reverse: 5′-GTCCACCACCCTGTTGCTGTAG-3′
- CYP1A2:
- Forward: 5′-TCAACCTCGTGAAGAGCAGCA-3′
- Reverse: 5′-GTCCTGGATACTGTTCTTGTTGAAGTC-3′
- CYP2E1:
- Forward: 5′-GACCAAAGGCCAGCCTTTTG-3′
- Reverse: 5′-GTTATTGTAAAGCTGGATCCAGGGG-3′
- CYP3A1:
- Forward: 5′-TCTGTGCAGAAGCATCGAGTG-3′
- Reverse: 5′-TGGGAGGTGCCTTATTGGG-3′
3.3.5. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Chinese Pharmacopoeia Commission, Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2010; Volume 1, p. 216.
- Ding, A.; Zhang, L.; Ding, J. Extraction and application of schizonepetin. Chinese Patent ZL01108186, 9 April 2001. [Google Scholar]
- Zhang, L.; Zhang, M.; Sun, E.; Ding, A. Anti-inflammatory, and antipyretic effects of schizonepetolide polylactie-co-glycolic acid nanoparticles. J. China Pharm. Univ 2008, 39, 433–436. [Google Scholar]
- Zhu, H.; Zhang, L.; Yu, B.; Zhang, L.; Ding, A. Study on general Pharmacology of schizonepetin. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 237–240. [Google Scholar]
- Liu, D.; Geng, T.; Zhang, L.; Yao, W.; Ding, A.; Shan, M. Acute and subacute toxicity and genotoxicity of schizonepetin, a naturally occurring monoterpene with antiviral activity. Food Chem. Toxicol 2012, 50, 2256–2262. [Google Scholar]
- Geng, T.; Sun, Y.; Yao, W.; Ding, A.; Zhang, L.; Guo, J.; Tang, Y. Pharmacokinetics and tissue distribution of schizonepetin in rats. Fitoterapia 2011, 82, 1110–1117. [Google Scholar]
- Kim, E.; Lee, S.; Jung, H.; Jung, H.; Yeo, C.; Shon, J.; Shin, J. Robust CYP2D6 genotype assay including copy number variation using multiplex single-base extension for Asian populations. Clin. Chim. Acta 2010, 411, 2043–2048. [Google Scholar]
- Nebert, D.W.; Russell, D.W. Clinical importance of the cytochromes P450. Lancet 2002, 360, 1155–1162. [Google Scholar]
- Hanapi, N.A.; Azizi, J.; Ismail, S.; Mansor, S.M. Evaluation of selected Malaysian medicinal plants on phase I drug metabolizing enzymes, CYP2C9, CYP2D6 and CYP3A4 activities in vitro. Int. J. Pharmacol 2010, 6, 494–499. [Google Scholar]
- Rodriguez-Antona, C.; Ingelman-Sundberg, M. Cytochrome P450 pharmacogenetics and cancer. Oncogene 2006, 25, 1679–1691. [Google Scholar]
- Nebert, D.W.; Dalton, T.P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat. Rev. Cancer 2006, 6, 947–960. [Google Scholar]
- Bjornsson, T.D.; Callaghan, J.T.; Einolf, H.J.; Fischer, V.; Gan, L.; Grimm, S.; Kao, J.; King, S.P.; Miwa, G.; Ni, L.; et al. The conduct of in vitro and in vivo drug-drug interaction studies: A Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab. Dispos 2003, 31, 815–832. [Google Scholar]
- Paolini, M.; Biagi, G.L.; Bauer, C.; Cantelliforti, G. Cocktail strategy: Complications and limitations. J. Clin. Pharmacol 1993, 33, 1011–1012. [Google Scholar]
- Zhou, H.H.; Tong, Z.; McLeod, J.F. “Cocktail” approaches and strategies in drug development: Valuable tool or flawed science? J. Clin. Pharmacol 2004, 44, 120–134. [Google Scholar]
- Hiratsuka, M. In vitro assessment of the allelic variants of cytochrome P450. Drug Metab. Pharmacokinet 2012, 27, 68–84. [Google Scholar]
- Kawase, A.; Fujii, A.; Negoro, M.; Akai, R.; Ishikubo, M.; Komura, H.; Iwaki, M. Differences in cytochrome P450 and nuclear receptor mRNA levels in liver and small intestines between SD and DA rats. Drug Metab. Pharmacokinet 2008, 23, 196–206. [Google Scholar]
- Lu, S.K.; Callahan, S.M.; Brunner, L.J. Suppression of hepatic CYP3A1/2 and CYP2C11 by cyclosporine is not mediated by altering growth hormone levels. J. Pharmacol. Exp. Ther 2003, 305, 331–337. [Google Scholar]
- Mustajoki, P.; Mustajoki, S.; Rautio, A.; Arvela, P.; Pelkonen, O. Effects of heme arginate on cytochrome P450-mediated metabolism of drugs in patients with variegate porphyria and in healthy men. Clin. Pharmacol. Ther 1994, 56, 9–13. [Google Scholar]
- Yao, X.; Wang, B.; Gu, Y.; Li, Y. Effects of bicyclol on the activity and expression of CYP450 enzymes of rats after partial hepatectomy. Acta Pharm. Sinica 2011, 46, 656–663. [Google Scholar]
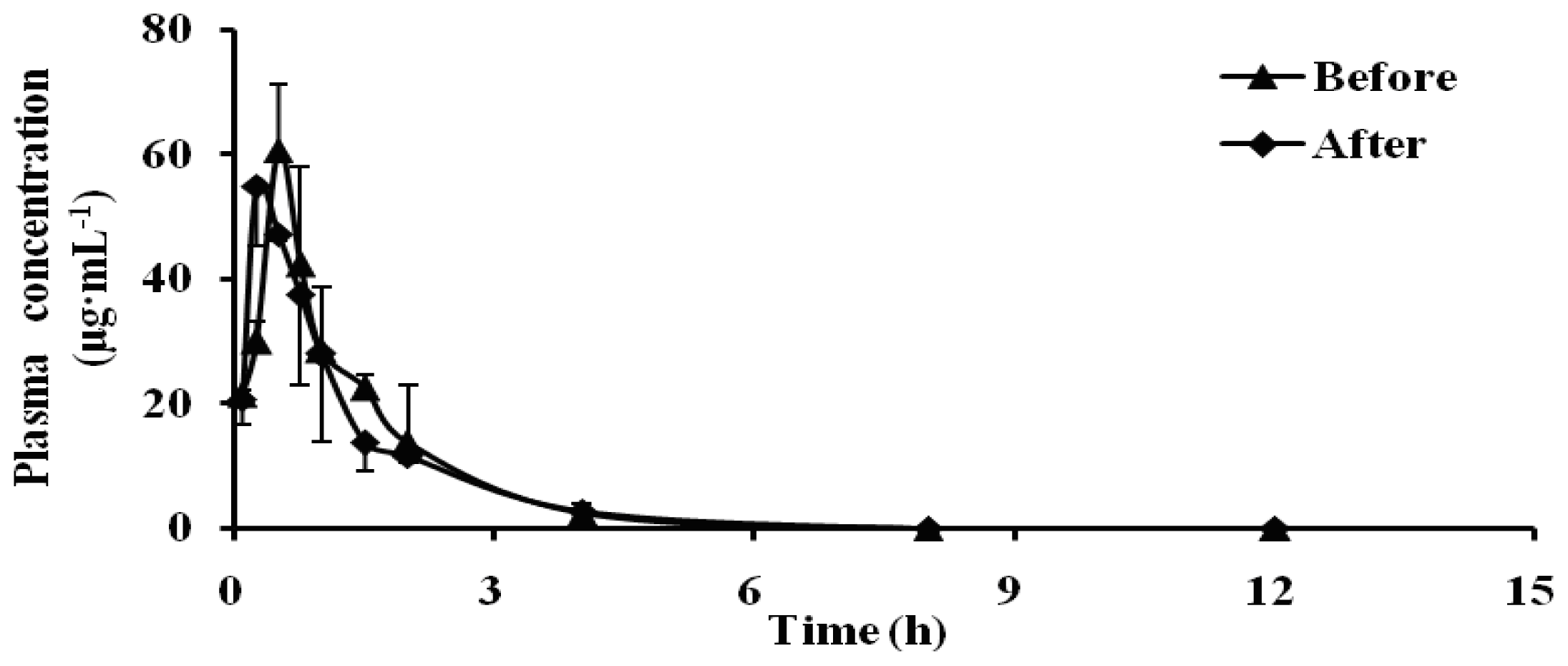
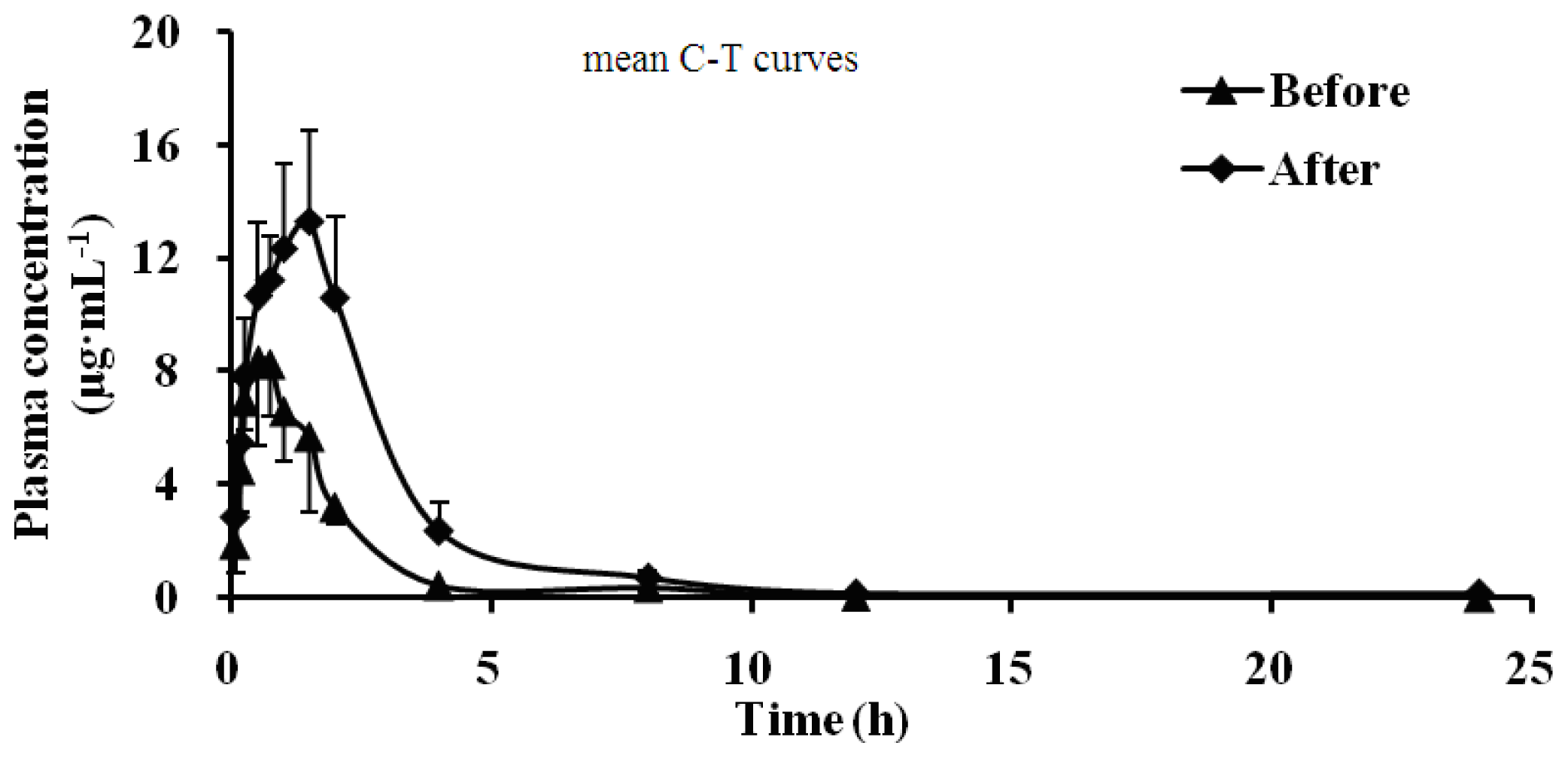

| Parameter/Unit | Dapsone | |
|---|---|---|
| Before | After | |
| Cmax/μg·mL−1 | 17.72 ± 3.19 | 10.13 ± 2.12 |
| Tmax/h | 2.10 ± 0.22 | 0.85 ± 0.14 |
| t1/2/h | 15.77 ± 2.98 | 19.44 ± 5.24 |
| AUC0–t/μg·h·mL−1 | 272.6 ± 48.89 | 150.5 ± 14.12 |
| AUC0–∞/μg·h·mL−1 | 425.3 ± 98.30 | 268.6 ± 56.34 |
| MRT0–t/h | 10.29 ± 0.18 | 10.19 ± 0.64 |
| MRT0–∞/h | 23.35 ± 3.91 | 28.53 ± 7.85 |
| CL/L·h−1 | 32.68 ± 6.95 | 53.20 ± 10.04 |
| Vz/L | 0.73 ± 0.17 | 1.45 ± 0.32 |
| Parameter/Unit | Phenacetin | Acetaminophen | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Cmax/μg·mL−1 | 30.94 ± 0.39 | 41.72 ± 0.38 | 23.4 ± 2.83 | 29.90 ± 6.60 |
| Tmax/h | 0.31 ± 0.13 | 0.25 ± 0.00 | 1.13 ± 0.25 | 1.10 ± 0.22 |
| t1/2/h | 2.32 ± 0.56 | 2.38 ± 0.57 | 7.79 ± 1.38 | 1.67 ± 0.25 |
| AUC0–t/μg·h·mL−1 | 67.48 ± 4.87 | 90.35 ± 19.21 | 143.5 ± 32.55 | 89.45 ± 23.91 |
| AUC0–∞/μg·h·mL−1 | 70.43 ± 7.97 | 91.08 ± 18.99 | 162.1 ± 39.81 | 90.68 ± 24.03 |
| MRT0–t/h | 2.34 ± 0.36 | 2.52 ± 0.36 | 7.33 ± 0.17 | 2.40 ± 0.26 |
| MRT0–∞/h | 2.78 ± 0.52 | 2.72 ± 0.23 | 10.52 ± 1.16 | 2.54 ± 0.21 |
| CL/L·h−1 | 1.11 ± 0.16 | 0.92 ± 0.14 | 0.49 ± 0.10 | 0.95 ± 0.25 |
| Vz/L | 3.65 ± 0.76 | 3.25 ± 1.18 | 5.62 ± 1.31 | 2.25 ± 0.39 |
| Parameter/Unit | Chlorzoxazone | |
|---|---|---|
| Before | After | |
| Cmax/μg·mL−1 | 20.20 ± 4.30 | 35.56 ± 6.96 |
| Tmax/h | 1.44 ± 0.52 | 0.25 ± 0.00 |
| t1/2/h | 3.32 ± 0.89 | 3.35 ± 1.13 |
| AUC0–t/μg·h·mL−1 | 120.3 ± 16.75 | 165.0 ± 40.03 |
| AUC0–∞/μg·h·mL−1 | 133.5 ± 26.55 | 181.8 ± 41.34 |
| MRT0–t/h | 4.39 ± 0.50 | 3.94 ± 0.25 |
| MRT0–∞/h | 5.58 ± 0.62 | 5.20 ± 0.59 |
| CL/L·h−1 | 0.11 ± 0.03 | 0.08 ± 0.03 |
| Vz/L | 0.52 ± 0.06 | 0.42 ± 0.25 |
| Parameter/Unit | Omeprazole | |
|---|---|---|
| Before | After | |
| Cmax/μg·mL−1 | 60.76 ± 10.53 | 55.69 ± 9.71 |
| Tmax/h | 0.42 ± 0.14 | 0.38 ± 0.18 |
| t1/2/h | 0.76 ± 0.25 | 1.03 ± 0.25 |
| AUC0–t/μg·h·mL−1 | 71.11 ± 20.06 | 67.13 ± 15.97 |
| AUC0–∞/μg·h·mL−1 | 74.02 ± 20.48 | 71.66 ± 19.43 |
| MRT0–t/h | 1.18 ± 0.10 | 1.14 ± 0.07 |
| MRT0–∞/h | 1.34 ± 0.16 | 1.40 ± 0.23 |
| CL/L·h−1 | 0.09 ± 0.02 | 0.09 ± 0.02 |
| Vz/L | 0.10 ± 0.05 | 0.13 ± 0.01 |
| Parameter/Unit | Metoprolol | |
|---|---|---|
| Before | After | |
| Cmax/μg·mL−1 | 9.47 ± 1.74 | 14.34 ± 2.57 |
| Tmax/h | 0.50 ± 0.20 | 1.33 ± 0.29 |
| t1/2/h | 1.32 ± 0.58 | 2.91 ± 1.61 |
| AUC0–t/μg·h·mL−1 | 15.89 ± 2.03 | 37.89 ± 2.86 |
| AUC0–∞/μg·h·mL−1 | 16.38 ± 1.95 | 40.52 ± 5.05 |
| MRT0–t/h | 1.75 ± 0.42 | 3.01 ± 1.09 |
| MRT0–∞/h | 2.01 ± 0.52 | 3.71 ± 1.34 |
| CL/L·h−1 | 1.04 ± 0.18 | 0.47 ± 0.07 |
| Vz/L | 2.05 ± 1.05 | 2.07 ± 1.29 |
| Probe substrate | Mobile phase | ë | Internal standard |
|---|---|---|---|
| Dapsone | A (methanol):B (water, 0.02% Triethylamine, pH 3.0) = 80:20 (v/v) | 292 m | tinidazole 0.25 mg·mL−1 |
| Phenacetin | A (methanol):B (water), gradient elution, 0–4 min (35% A, 65% B), 5–20 min (45% A, 55% B), 21–23 min (35% A, 65% B) | 245 m | 4-acetaminophen 0.2 mg·mL−1 |
| Chlorzoxazone | methanol:water = 48:52 (v/v) | 278 m | omeprazole 0.25 mg·mL−1 |
| Omeprazole | A (methanol):B (water), gradient elution, 0–7 min (52% A, 48% B), 8–12 min (40% A, 60% B), 13–17 min (52% A, 48% B) | 300 m | coumarin 0.1 mg·mL−1 |
| Metoprolol | A (methanol):B (water, 0.02% Triethylamine, pH 3.5) = 40:60 (v/v) | 225 m | phenacetin 0.25 mg·mL−1 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bao, B.; Geng, T.; Cao, Y.; Yao, W.; Zhang, L.; Ding, A. Effects of Schizonepetin on Activity and mRNA Expression of Cytochrome P450 Enzymes in Rats. Int. J. Mol. Sci. 2012, 13, 17006-17018. https://doi.org/10.3390/ijms131217006
Bao B, Geng T, Cao Y, Yao W, Zhang L, Ding A. Effects of Schizonepetin on Activity and mRNA Expression of Cytochrome P450 Enzymes in Rats. International Journal of Molecular Sciences. 2012; 13(12):17006-17018. https://doi.org/10.3390/ijms131217006
Chicago/Turabian StyleBao, Beihua, Ting Geng, Yudan Cao, Weifeng Yao, Li Zhang, and Anwei Ding. 2012. "Effects of Schizonepetin on Activity and mRNA Expression of Cytochrome P450 Enzymes in Rats" International Journal of Molecular Sciences 13, no. 12: 17006-17018. https://doi.org/10.3390/ijms131217006
APA StyleBao, B., Geng, T., Cao, Y., Yao, W., Zhang, L., & Ding, A. (2012). Effects of Schizonepetin on Activity and mRNA Expression of Cytochrome P450 Enzymes in Rats. International Journal of Molecular Sciences, 13(12), 17006-17018. https://doi.org/10.3390/ijms131217006




