Effects of Sesamin on Streptozotocin (STZ)-Induced NIT-1 Pancreatic β-Cell Damage
Abstract
:1. Introduction
2. Results and Discussion
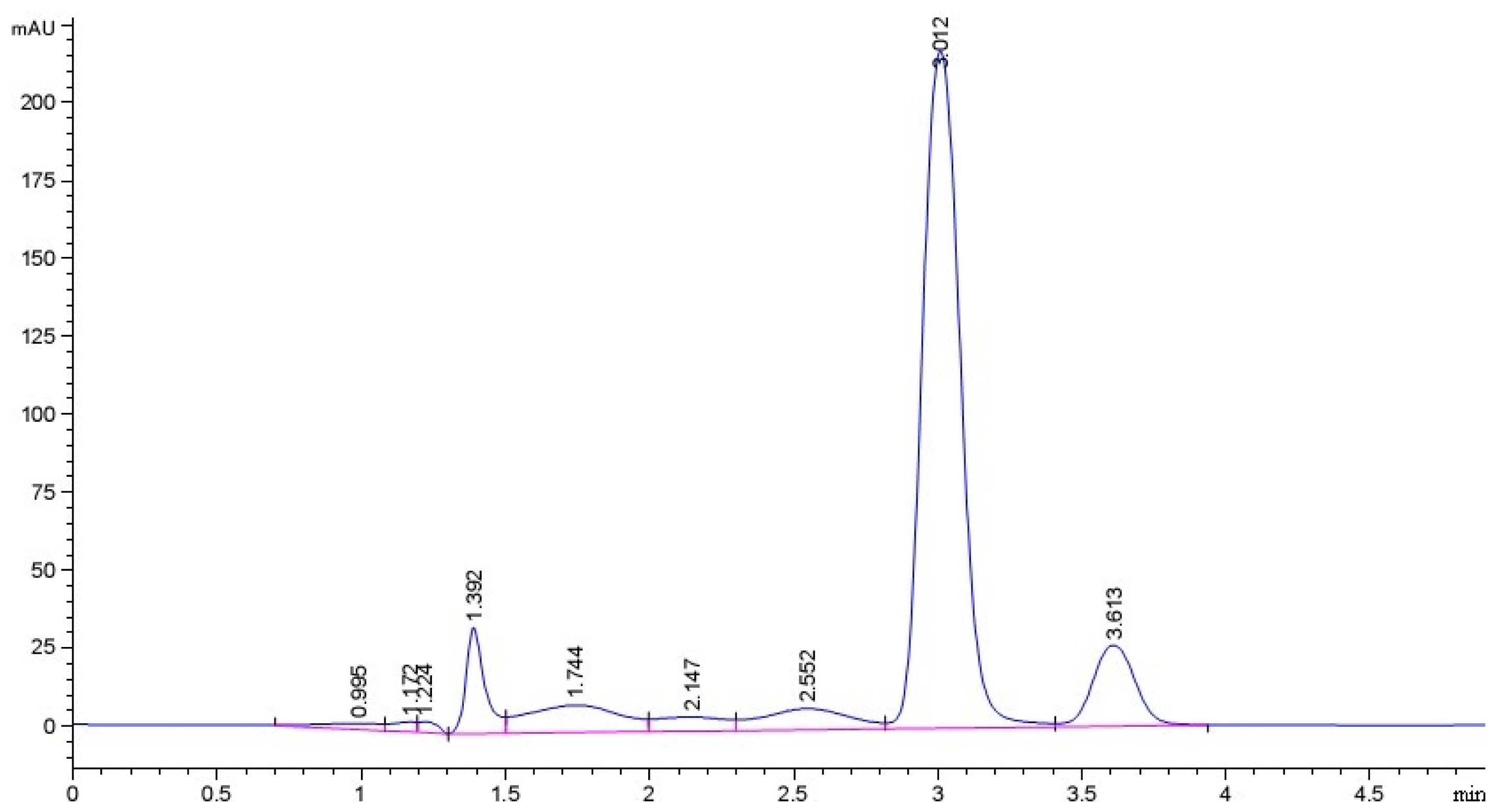
2.1. Purity of Sesamin (SES) Sample
2.2. Effect of Cytotoxicity of Streptozotocin (STZ) on the Pancreatic NIT-1 Cells
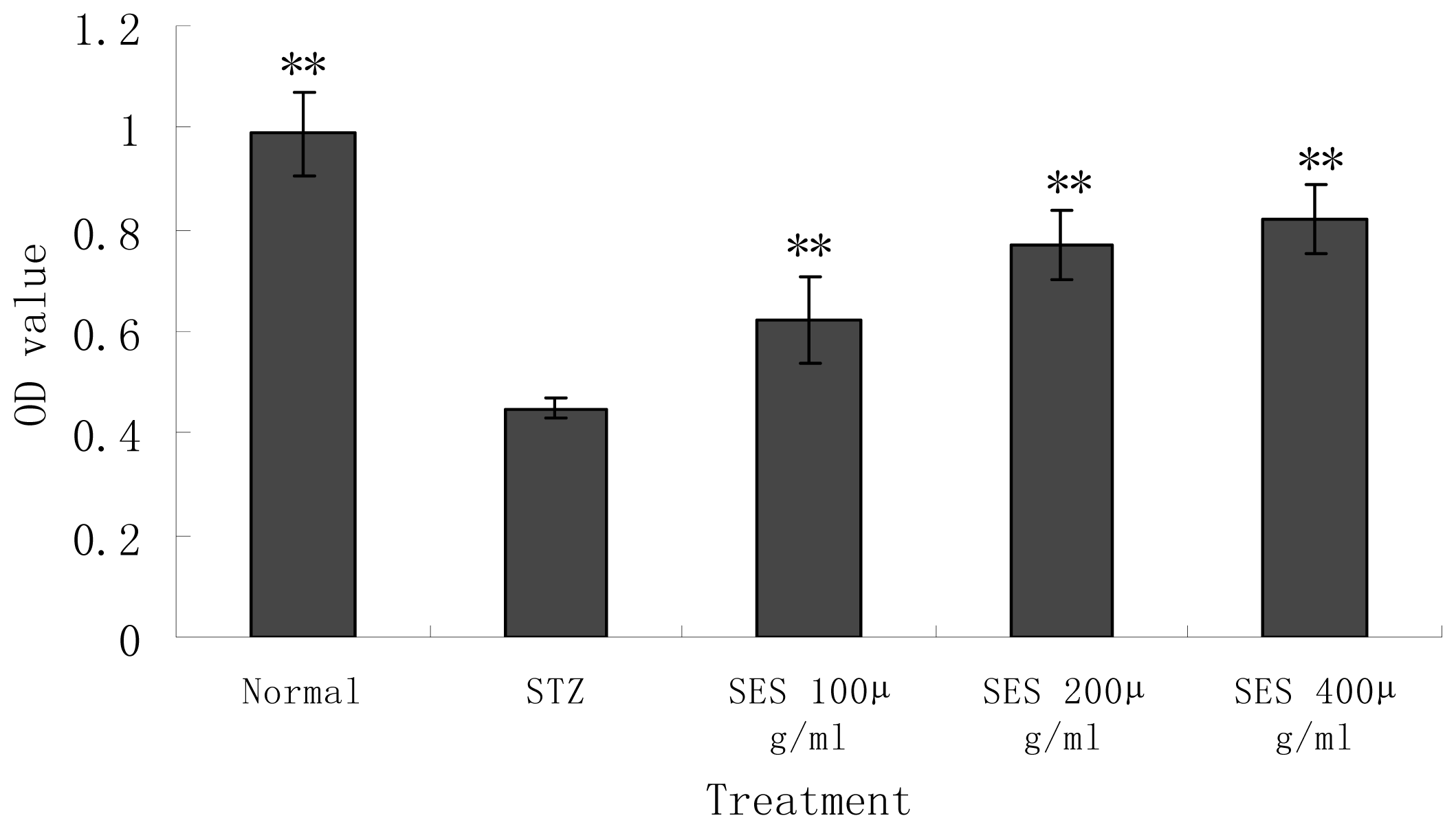
2.3. Effect of SES on the Viability of NIT-1 Cells Damaged by STZ
2.4. Effect of SES on Insulin Secretion by NIT-1 Cells Damaged by STZ
2.5. Effect of SES on Levels of SOD, GSHpx, GSH and MDA in NIT-1 Cells
2.6. Effect of SES on Levels of Nitric Oxide (NO), NOS, iNOS in NIT-1 Cells
2.7. Effect of SES on the Microscopic Observation of NIT-1 Cells Damaged by STZ
3. Experimental Section
3.1. Preparation and Purity of Sesamin (SES)
3.2. Main Reagents
3.3. NIT-1 Cell Culture
3.4. Cytotoxity of Streptozotocin (STZ)
3.5. MTT Assay
3.6. Experimental Design
3.7. Microscopic Observation
3.8. Biochemical Measurements
3.9. Statistical Analysis
4. Conclusions
References
- Kanu, P.J.; Kerui, Z.; Jestina, B.K.; Zhou, H.M.; Qian, H.F.; Zhu, K.X. Biologically active components and nutraceuticals in sesame and related products: A review and prospect. Trends Food Sci. Technol 2007, 18, 599–608. [Google Scholar]
- Zhou, J.C.; Feng, D.W.; Zheng, G.S. Extraction of sesamin from sesame oil using macro-porous resin. J. Food Eng 2010, 100, 289–293. [Google Scholar]
- Yasuko, F.; Toshihiko, O.; Mitsuo, N. Studies on antioxidative substances in sesame seed. Agric. Biol. Chem 1983, 49, 301–306. [Google Scholar]
- Dong, Y.; Gao, Y.; Xu, B. Extraction of antioxidant from sesame cake and its activity study. Food Sci 2007, 28, 44–47. [Google Scholar]
- Jin, Q.Z.; Liu, Y.F.; Wang, X.G.; Dai, H.P. Sesamin as a natural antioxidant. J. Chin. Cereals Oil Assoc 2005, 20, 89–93. [Google Scholar]
- Bian, H.L. Effect of sesamin on immune function and proliferating cell nuclear antigen in S180 sarcoma-bearing mice. China J. Mod. Med 2008, 18, 3411–3417. [Google Scholar]
- Hirose, N.; Doi, F.; Ueki, T.; Akazawa, K.; Chijiiwa, K.; Sugano, M.; Akimoto, K.; Shimizu, S.; Yamada, H. Suppressive effect of sesamin against 7,12-dimethylbenz[a]-anthracene induced rat mammary carcinogenesis. Anticancer Res 1992, 12, 1259–1265. [Google Scholar]
- Wei, Y.J.; Bian, H.L.; Zhao, C.F. Inhibitory effect of sesamin on tumor growth and mechanism in H22 hepatoma-bearing mice. Shangdong Med 2008, 48, 25–27. [Google Scholar]
- Matsumura, Y.; Kita, S.; Tanida, Y.; Taguchi, Y.; Morimoto, S.; Akimoto, K.; Tanaka, T. Antihypertensive effect of sesamin. III. Protection against development and maintenance of hypertension in stroke-prone spontaneously hypertensive rats. Biol. Pharm. Bull 1998, 21, 469–473. [Google Scholar]
- Wang, W.S.; Song, J.G. The protective effects of sesamin against liver damage in rodents. Pharmacol. Clin. Chin. Mater. Med 2006, 22, 27–32. [Google Scholar]
- Hirata, F.; Fujita, K.; Ishikura, Y.; Hosoda, K.; Ishikawa, T.; Nakamura, H. Hypocholesterolemic effect of sesame lignan in humans. Atherosclerosis 1996, 122, 135–136. [Google Scholar]
- Hirose, N.; Inoue, T.; Nishihara, K.; Sugano, M.; Akimoto, K.; Shimizu, S.; Yamada, H. Inhibition of cholesterol absorption and synthesis in rats by sesamin. J. Lipid Res 1991, 32, 629–638. [Google Scholar]
- Ashakumary, L.; Rouyer, I.; Takahashi, Y.; Ide, T.; Fukuda, N.; Aoyama, T.; Hashimoto, T.; Mizugaki, M.; Sugano, M. Sesamin, a sesame lignan, is a potent inducer of hepatic fatty acid oxidation in the rat. Metabolism 1999, 48, 1303–1313. [Google Scholar]
- Takashi, I.; Lakshmikuttyamma, A.; Yoko, T.; Masayo, K.; Nobuhiro, F.; Michihiro, S. Sesamin, a sesame lignan, decreases fatty acid synthesis in rat liver accompanying the down-regulation of sterol regulatory element binding protein-1. Biochim. Biophys. Acta 2001, 1534, 1–13. [Google Scholar]
- Nobuo, T.; Ayako, K.; Ichiro, M.; Keiko, A.; Yoshinobu, K. Modulating effect of sesamin, a functional lignan in sesame seeds, on the transcription levels of lipid- and alcohol-metabolizing enzymes in rat liver: A DNA microarray study. Biosci. Biotech. Biochem 2005, 69, 179–188. [Google Scholar]
- Hamaguchi, K.; Gaskins, H.R.; Leiter, E.H. NIT-1, a pancreatic beta-cell line established from a transgenic NOD/Lt mouse. Diabetes 1991, 40, 842–849. [Google Scholar]
- Luo, J.; Quan, J.; Joyce, T.; Christina, K.; Cynthia, S.; Richard, H.; Gerald, M. Nongenetic mouse models of non-insulin-dependent diabetes mellitus. Metabolism 1998, 47, 663–668. [Google Scholar]
- Jia, Y.J.; Xin, Z.L.; Hu, H.T.; Ren, H.M.; Wang, W.X. Melatonin protects β-cell from streptozotocin induced injury. J. Xi’an Med. Univ 2000, 21, 13–15. [Google Scholar]
- Zhang, H.N.; He, J.H.; Yuan, L.; Lin, Z.B. In vitro and in vivo protective effect of Ganoderma lucidum polysaccharides on alloxan-induced pancreatic islets damage. Life Sci 2003, 73, 2307–2319. [Google Scholar]
- Hirotada, K.; Noriko, K.; Hiroaki, N. Activities of polysaccharides obtained from Grifola frondosa on insulin-dependent diabetes mellitus induced by streptozotocin in mice. Mycoscience 2000, 41, 473–480. [Google Scholar]
- Ji, H.P.; Ji, H.K.; Won, H.K. Association of the endothelial nitric oxide synthase(ecNOS) gene polymorphism with carotidatherosclerosis in type 2 diabetes. Diabetes Res. Clin. Pract 2006, 72, 322–327. [Google Scholar]
- Song, I.K.; Kap, S.K.; Seok, J.S.; Myung, S.K.; Dae, Y.K.; Sun, L.K.; Cheorl, H.K. Anti-inflammatory effect of Ulmus davidiana Planch (Ulmaceae) on collagen-induced inflammation in rats. Environ. Toxicol. Pharmacol 2007, 23, 102–110. [Google Scholar]



| Group | Dose(μg mL−1) | SOD(U/mgpr) | GSHpx(U/mgpr) | GSH(mg/gpr) | MDA(nmol/mgpr) |
|---|---|---|---|---|---|
| Normal control | - | 16.20 ± 2.38 * | 15.57 ± 2.00 ** | 38.71 ± 4.92 * | 0.469 ± 0.061 ** |
| STZ model | - | 11.42 ± 1.68 | 11.07 ± 1.44 | 30.82 ± 4.02 | 0.717 ± 0.128 |
| SES | 100 | 13.69 ± 1.56 | 13.17 ± 1.18 * | 35.65 ± 3.92 | 0.571 ± 0.079 |
| 200 | 15.28 ± 1.30 ** | 13.90 ± 1.53 * | 38.45 ± 4.42 * | 0.456 ± 0.063 ** | |
| 400 | 16.54 ± 2.59 ** | 14.68 ± 2.63 * | 40.06 ± 4.18 * | 0.458 ± 0.080 ** |
| Group | Dose(μg mL−1) | NO(μmol/L) | NOS(U/mL) | iNOS(U/mL) |
|---|---|---|---|---|
| Normal control | - | 56.94 ± 5.73 ** | 1.92 ± 0.30 ** | 0.64 ± 0.20 * |
| STZ model | - | 89.34 ± 5.12 | 3.01 ± 0.35 | 1.00 ± 0.23 |
| SES | 100 | 76.79 ± 4.42 ** | 2.50 ± 0.25 * | 0.86 ± 0.28 |
| 200 | 63.83 ± 5.26 ** | 2.21 ± 0.35 * | 0.75 ± 0.30 | |
| 400 | 58.43 ± 3.31 ** | 2.10 ± 0.18 ** | 0.61 ± 0.20 * |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lei, H.; Han, J.; Wang, Q.; Guo, S.; Sun, H.; Zhang, X. Effects of Sesamin on Streptozotocin (STZ)-Induced NIT-1 Pancreatic β-Cell Damage. Int. J. Mol. Sci. 2012, 13, 16961-16970. https://doi.org/10.3390/ijms131216961
Lei H, Han J, Wang Q, Guo S, Sun H, Zhang X. Effects of Sesamin on Streptozotocin (STZ)-Induced NIT-1 Pancreatic β-Cell Damage. International Journal of Molecular Sciences. 2012; 13(12):16961-16970. https://doi.org/10.3390/ijms131216961
Chicago/Turabian StyleLei, Hong, Juncheng Han, Qin Wang, Shuzhen Guo, Hanju Sun, and Xiaoxiang Zhang. 2012. "Effects of Sesamin on Streptozotocin (STZ)-Induced NIT-1 Pancreatic β-Cell Damage" International Journal of Molecular Sciences 13, no. 12: 16961-16970. https://doi.org/10.3390/ijms131216961
APA StyleLei, H., Han, J., Wang, Q., Guo, S., Sun, H., & Zhang, X. (2012). Effects of Sesamin on Streptozotocin (STZ)-Induced NIT-1 Pancreatic β-Cell Damage. International Journal of Molecular Sciences, 13(12), 16961-16970. https://doi.org/10.3390/ijms131216961





