Histone Deacetylase (HDAC) Inhibitors Down-Regulate Endothelial Lineage Commitment of Umbilical Cord Blood Derived Endothelial Progenitor Cells
Abstract
:1. Introduction
2. Results and Discussion
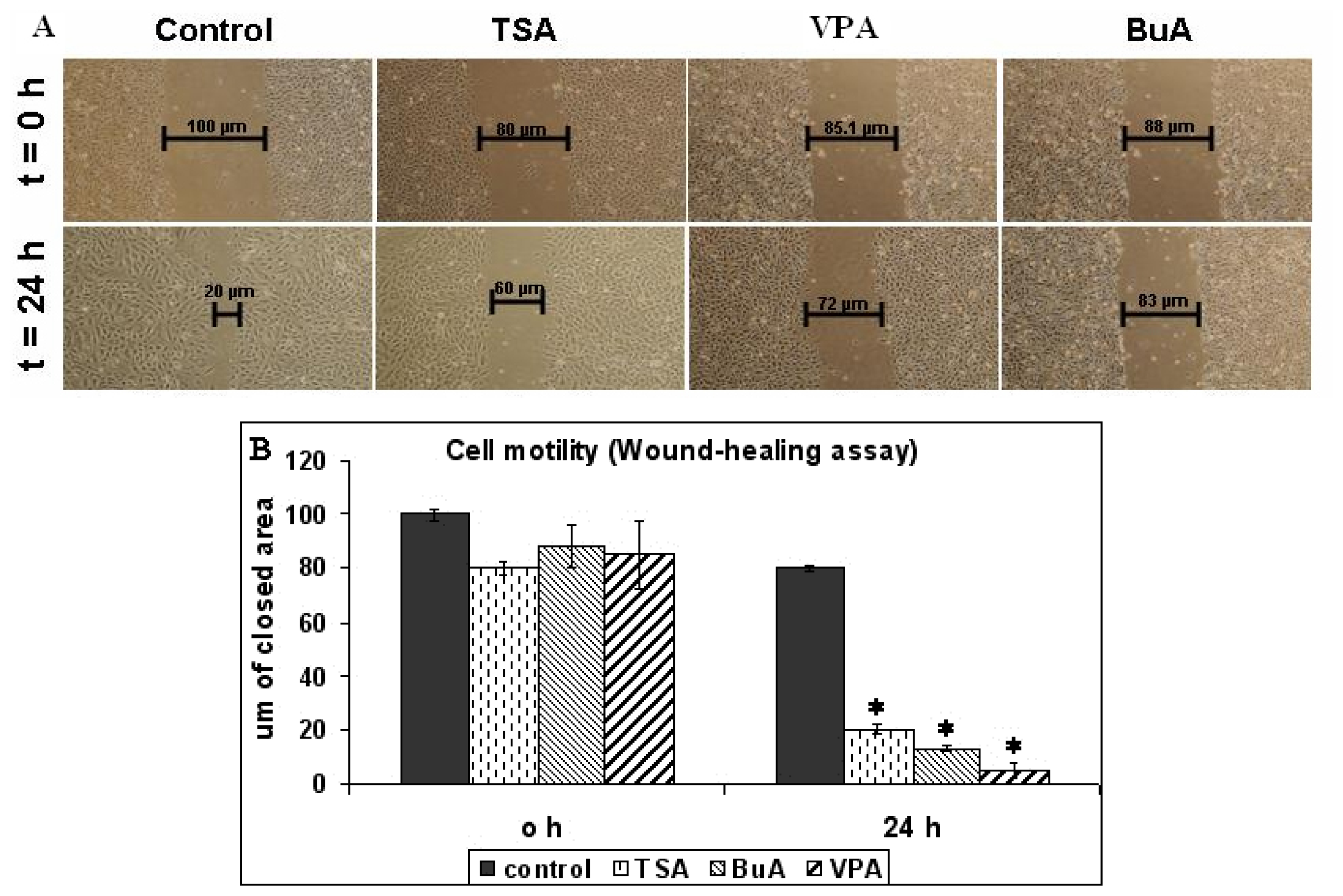
2.1. EPC Proliferation and Motility
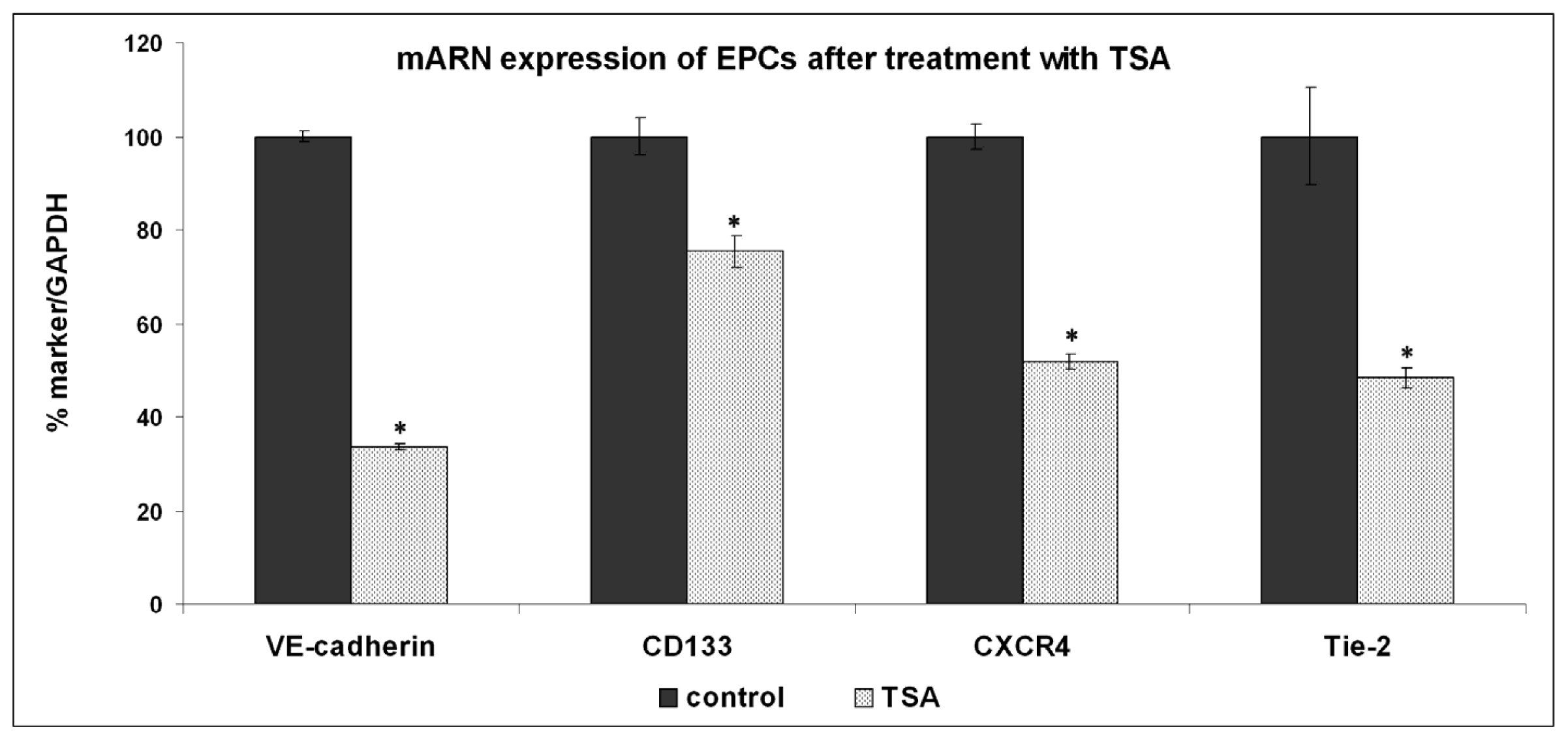
2.2. Expression of Molecules Involved in EPCs Differentiation
3. Experimental Section
3.1. Cell Culture
3.2. Cell Proliferation and Cytotoxicity Assay
3.3. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3.4. Real Time-PCR Quantification of Telomerase Activity
3.5. Flow Cytometry
3.6. In vitro Wound-Healing Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestsThe authors declare no conflicts of interests or competing financial.
References
- Jenuwein, T.; Allis, C.D. Translating the Histone Code. Science 2001, 293, 1074–1080. [Google Scholar]
- Li, B.; Carey, M.; Workman, J.L. The Role of Chromatin during Transcription. Cell 2007, 128, 707–719. [Google Scholar]
- Lee, J.S.; Smith, E.; Shilatifard, A. The Language of Histone Crosstalk. Cell 2010, 142, 682–685. [Google Scholar]
- Vincent, A.; Seuningen, V.I. Epigenetics, stem cells and epithelial cell fate. Differentiation 2009, 78, 99–107. [Google Scholar]
- Robertson, K.D. DNA methylation and human disease. Nat. Rev. Genet 2005, 6, 597–610. [Google Scholar]
- Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol 2009, 71, 241–260. [Google Scholar]
- Gan, Y.; Shen, Y.H.; Wang, J.; Wang, X.; Utama, B.; Wang, J.; Wang, X.L. Role of Histone Deacetylation in Cell-specific Expression of Endothelial Nitric-oxide Synthase. J. Biol. Chem 2005, 280, 16467–16475. [Google Scholar]
- Mariadason, J.M. HDACs and HDAC inhibitors in colon cancer. Epigenetics 2008, 3, 28–37. [Google Scholar]
- Witt, O.; Deubzer, H.E.; Milde, T.; Oehme, I. HDAC family: What are the cancer relevant targets? Cancer Lett 2009, 277, 8–21. [Google Scholar]
- Monneret, C. Histone deacetylase inhibitors. Eur. J. Med. Chem. 2005, 401–413. [Google Scholar]
- Lupu, M.; Khalil, M.; Andrei, E.; Iordache, F.; Pfannkuche, K.; Neef, K.; Georgescu, A.; Buzila, C.; Brockmeier, K.; Maniu, H.; et al. Integration Properties of Wharton’s Jelly-derived Novel Mesenchymal Stem Cells into Ventricular Slices of Murine Hearts. Cell. Physiol. Biochem 2011, 28, 63–76. [Google Scholar]
- Lupu, M.; Khalil, M.; Iordache, F.; Andrei, E.; Pfannkuche, K.; Spitkovsky, D.; Baumgartner, S.; Rubach, M.; AbdelRazik, H.; Buzila, C.; et al. Direct Contact of Umbilical Cord Blood Endothelial Progenitors with Living Cardiac Tissue is a Requirement for Vascular Tube-like Structures Formation. J. Cell. Mol. Med 2011, 15, 1914–1926. [Google Scholar]
- Iordache, F.; Iordache, C.; Pop, A.; Lupu, M.; Andrei, E.; Buzila, C.; Maniu, H. Effects of plant lectin and extracts on adhesion molecules of endothelial progenitors. Cent. Eur. J. Biol 2011, 6, 330–341. [Google Scholar]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar]
- Zhang, L.; Yang, R.; Han, Z.C. Transplantation of umbilical cord blood derived endothelial progenitor cells: A promising method of therapeutic revascularisation. Eur. J. Haematol 2006, 76, 1–8. [Google Scholar]
- Wada, T.; Kikuchi, J.; Nishimura, N.; Shimizu, R.; Kitamura, T.; Furukawa, Y. Expression levels of histone deacetylases determine the cell fate of hematopoietic progenitors. J. Biol. Chem 2009, 284, 30673–30683. [Google Scholar]
- Ohtani, K.; Dimmeler, S. Epigenetic regulation of cardiovascular differentiation. Cardiovasc. Res 2011, 90, 404–441. [Google Scholar]
- Dzau, V.J.; Gnecchi, M.; Pachori, A.S.; Morello, F.; Melo, L.G. Therapeutic Potential of Endothelial Progenitor Cells in Cardiovascular Diseases. Hypertension 2005, 46, 7–18. [Google Scholar]
- Cian, A.; Lacroix, L.; Douarre, C.; Temime-Smaali, N.; Trentesaux, C.; Riou, J.F.; Mergny, J.L. Targeting telomeres and telomerase. Biochimie 2008, 90, 131–155. [Google Scholar]
- Murasawa, S.; Llevadot, J.; Silver, M.; Isner, J.M.; Losordo, D.W.; Asahara, T. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation 2002, 106, 1133–1139. [Google Scholar]
- Yang, J.; Chang, E.; Cherry, A.M. Human endothelial cell life extension by telomerase expression. J. Biol. Chem 1999, 274, 26141–26148. [Google Scholar]
- Yoder, M.C. Defining human endothelial progenitor cells. J. Thromb. Haemost 2009, 7, 49–52. [Google Scholar]
- Duda, D.G.; Cohen, K.S.; Scadden, D.T.; Jain, R.K. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat. Protocol 2007, 2, 805–810. [Google Scholar]
- Rössig, L.; Urbich, C.; Brühl, T.; Dernbach, E.; Heeschen, C.; Chavakis, E.; Sasaki, K.; Aicher, D.; Diehl, F.; Seeger, F.; et al. Histone deacetylase activity is essential for the expression of HoxA9 and for endothelial commitment of progenitor cells. J. Exp. Med 2005, 201, 1825–1835. [Google Scholar]
- Xi, R.; Xie, T. Stem Cell Self-Renewal Controlled by Chromatin Remodeling Factors. Science 2005, 310, 1487–1489. [Google Scholar]
- Kretsovali, A.; Hadjimichael, C.; Charmpilas, N. Histone Deacetylase Inhibitors in Cell Pluripotency, Differentiation, and Reprogramming. Stem Cells Int. 2012. [Google Scholar] [CrossRef]
- Shi, G.; Gao, F.; Jin, Y. The regulatory role of histone deacetylase inhibitors in Fgf4 expression is dependent on the differentiation state of pluripotent stem cells. J. Cell. Physiol. 2011, 226, 3190–3196. [Google Scholar]
- Burba, I.; Colombo, G.I.; Staszewsky, L.I.; de Simone, M.; Devanna, P.; Nanni, S.; Avitabile, D.; Molla, F.; Cosentino, S.; Russo, I.; et al. Histone Deacetylase Inhibition Enhances Self Renewal and Cardioprotection by Human Cord Blood-Derived CD34+ Cells. PLoS One 2011, 6, e22158. [Google Scholar]
- Mottet, D.; Bellahcène, A.; Pirotte, S.; Waltregny, D.; Deroanne, C.; Lamour, V.; Lidereau, R.; Castronovo, V. Histone deacetylase 7 silencing alters endothelial cell migration, a key step in angiogenesis. Circ. Res 2007, 101, 1237–1246. [Google Scholar]
- Lee, J.H.; Hart, S.; Skalnik, D.G. Histone deacetylase activity is required for embryonic stem cell differentiation. Genesis 2004, 38, 32–38. [Google Scholar]
- Lagger, G.; O’Carroll, D.; Rembold, M.; Khier, H.; Tischler, J.; Weitzer, G.; Schuettengruber, B.; Hauser, C.; Brunmeir, R.; Jenuwein, T.; et al. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J 2002, 21, 2672–2681. [Google Scholar]
- Deroanne, C.F.; Bonjean, K.; Servotte, S.; Devy, L.; Colige, A.; Clausse, N.; Blacher, S.; Verdin, E.; Foidart, J.M.; Nusgens, B.V.; et al. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene 2002, 21, 427–436. [Google Scholar]
- Matouk, C.C.; Marsden, P.A. Epigenetic Regulation of Vascular Endothelial Gene Expression. Circ. Res 2008, 102, 873–887. [Google Scholar]
- Fischer, A.; Sananbenesi, F.; Wang, X.; Dobbin, M.; Tsai, L.H. Recovery of learning and memory is associated with chromatin remodelling. Nature 2007, 447, 178–182. [Google Scholar]



| Gene | GeneBank accession number | Sequences of oligonucleotide primers | Predicted size (pb) |
|---|---|---|---|
| GAPDH | NM_002046 | S: 5′-ACCACAGTCCATGCCATCAC-3′ A: 5′-TCCACCACCCTGTTGCTGTA-3′ | 450 |
| CXCR4 | NM_003467 | S: 5′-GATGACAGATATATCTGTGACCGC-3′ A: 5′-TTAGCTGGAGTGAAAACTTGAAGA-3′ | 519 |
| Tie-2 | NM_000459 | S: 5′-CATACTGGGGAAAGCAATGAAAC-3′ A: 5′-ACCACTGTTTTTCACCTTCCAAA-3′ | 281 |
| VE-cadherin | NM_001795 | A: 5′-CTTTGCCTCCAGGCAGATAG-3′ S: 5′-CCTTGGGATAGCAAACTCCA-3′ | 283 |
| CD133 | NM_006017 | S: 5′-CAGTCTGACCAGCGTGAAAA-3′ A: 5′-GGCCATCCAAATCTGTCCTA-3′ | 200 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iordache, F.; Buzila, C.; Constantinescu, A.; Andrei, E.; Maniu, H. Histone Deacetylase (HDAC) Inhibitors Down-Regulate Endothelial Lineage Commitment of Umbilical Cord Blood Derived Endothelial Progenitor Cells. Int. J. Mol. Sci. 2012, 13, 15074-15085. https://doi.org/10.3390/ijms131115074
Iordache F, Buzila C, Constantinescu A, Andrei E, Maniu H. Histone Deacetylase (HDAC) Inhibitors Down-Regulate Endothelial Lineage Commitment of Umbilical Cord Blood Derived Endothelial Progenitor Cells. International Journal of Molecular Sciences. 2012; 13(11):15074-15085. https://doi.org/10.3390/ijms131115074
Chicago/Turabian StyleIordache, Florin, Cosmin Buzila, Andrei Constantinescu, Eugen Andrei, and Horia Maniu. 2012. "Histone Deacetylase (HDAC) Inhibitors Down-Regulate Endothelial Lineage Commitment of Umbilical Cord Blood Derived Endothelial Progenitor Cells" International Journal of Molecular Sciences 13, no. 11: 15074-15085. https://doi.org/10.3390/ijms131115074
APA StyleIordache, F., Buzila, C., Constantinescu, A., Andrei, E., & Maniu, H. (2012). Histone Deacetylase (HDAC) Inhibitors Down-Regulate Endothelial Lineage Commitment of Umbilical Cord Blood Derived Endothelial Progenitor Cells. International Journal of Molecular Sciences, 13(11), 15074-15085. https://doi.org/10.3390/ijms131115074




