Effects of Acidic Polysaccharides from Gastrodia Rhizome on Systolic Blood Pressure and Serum Lipid Concentrations in Spontaneously Hypertensive Rats Fed a High-Fat Diet
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Acidic Polysaccharides from Gastrodia Rhizome
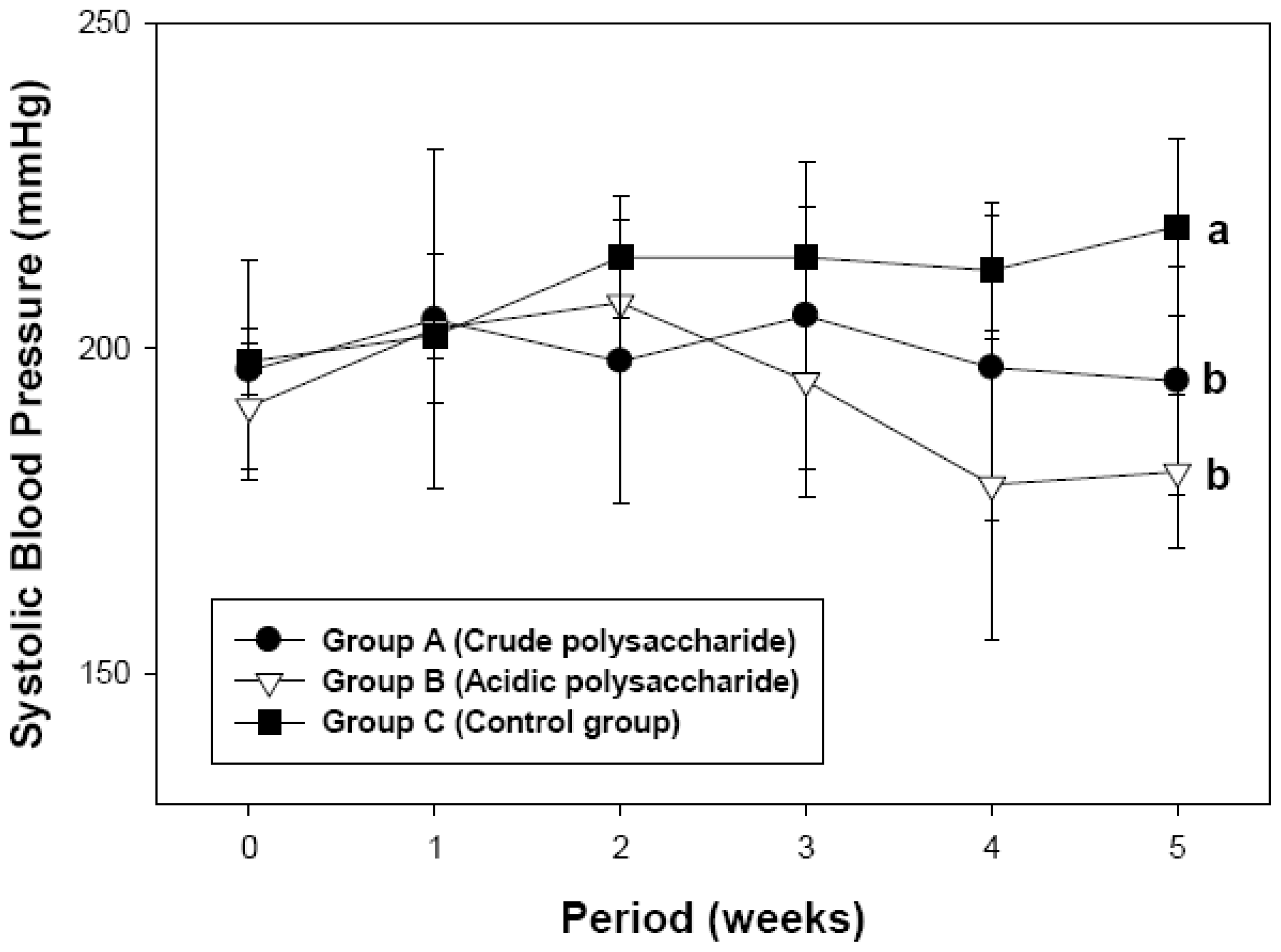
2.2. Antihypertensive Effect of Acidic Polysaccharides from Gastrodia Rhizome
2.3. Effects of Acidic Polysaccharides from Gastrodia Rhizome on Serum Lipid Levels
3. Experimental Section
3.1. Materials
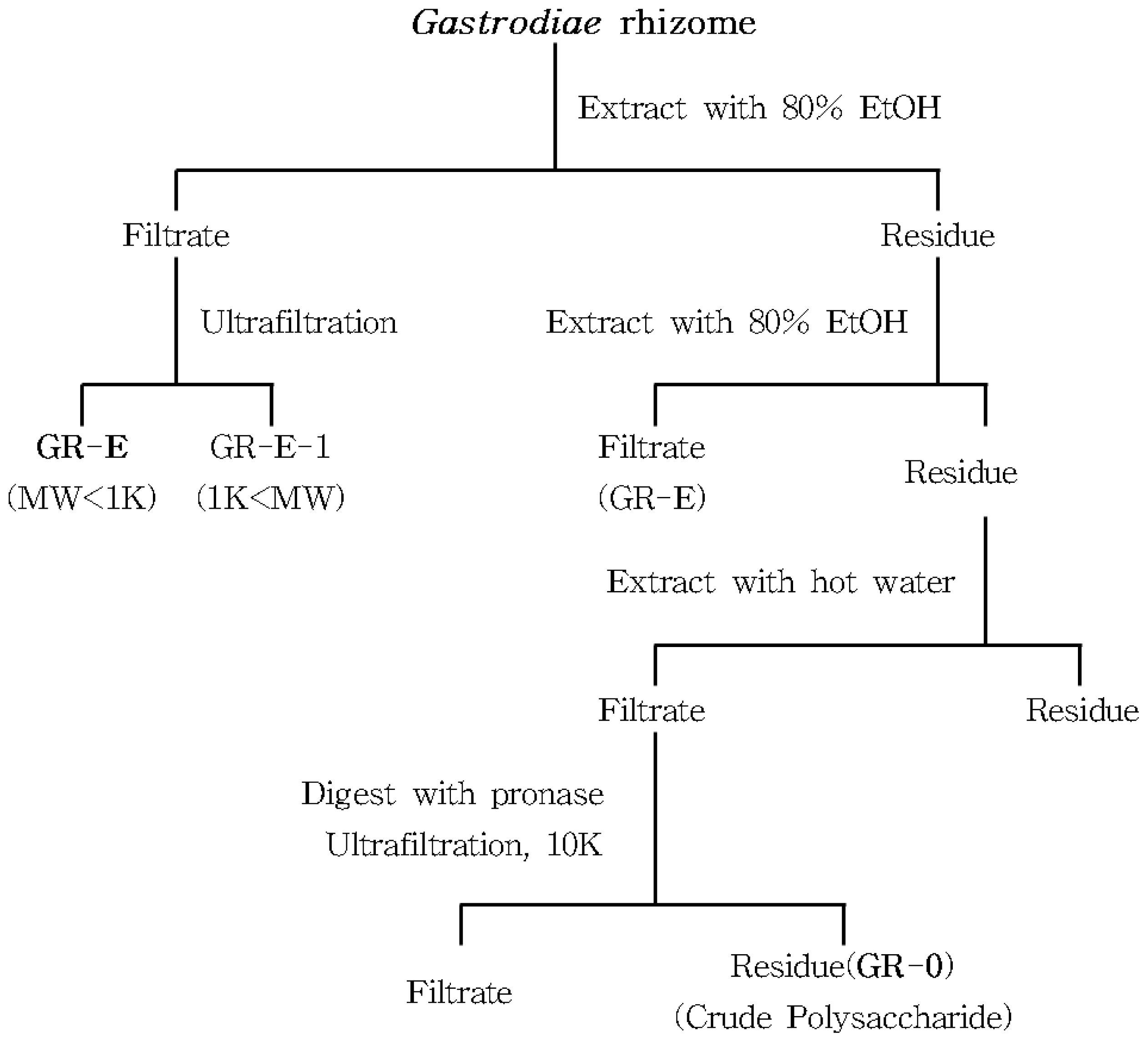
3.2. Preparation of Crude and Acidic Polysaccharides
3.3. Determination of Acidic Polysaccharides Profiles
3.4. Animal Experiments and Diet
3.5. Blood Pressure Measurements
3.6. Blood Chemistry
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Padmanabhan, S.; Paul, L.; Dominczak, A.F. The pharmacogenomics of anti-hypertensive therapy. Pharmaceuticals 2010, 3, 1779–1791. [Google Scholar]
- Peters, P.G.; Alessio, H.M.; Hagerman, A.E.; Ashton, T.; Nagy, S.; Wiley, R.L. Short-term isometric exercise reduces systolic blood pressure in hypertensive adults: Possible role of reactive oxygen species. Int. J. Cardiol 2006, 110, 199–205. [Google Scholar]
- Roberts, C.K.; Barnard, R.J. Effects of exercise and diet on chronic disease. J. Appl. Physiol 2005, 98, 3–30. [Google Scholar]
- Galloway, J.M. The epidemiology of atherosclerosis and its risk factors among Native Americans. Curr. Diab. Rep 2002, 2, 274–81. [Google Scholar]
- Sacks, F.M.; Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A dietary approach to prevent hypertension: A review of the Dietary Approaches to Stop Hypertension (DASH) study. Clin. Cardiol 1999, 22, III6–10. [Google Scholar]
- Craig, W.J. Nutrition concerns and health effects of vegetarian diets. Nutr. Clin. Pract 2010, 25, 613–620. [Google Scholar]
- Xie, J.T.; Wu, J.A.; Mehendale, S.; Aung, H.H.; Yuan, C.S. Anti-hyperglycemic effect of the polysaccharides fraction from American ginseng berry extract in ob/ob mice. Phytomedicine 2004, 11, 182–187. [Google Scholar]
- Miyazawa, N.; Okazaki, M.; Ohga, S. Antihypertensive effect of Pleurotus nebrodensis in spontaneously hypertensive rats. J. Oleo Sci 2008, 57, 675–681. [Google Scholar]
- Hong, H.D.; Shim, E.J.; Kim, K.I.; Choi, S.Y.; Han, C.K. Effect of Gastrodia elata Blume components on systolic blood pressure and serum lipid concentrations in spontaneously hypertensive rats fed high fat diet. J. Korean Soc. Food Sci. Nutr 2007, 36, 174–179. [Google Scholar]
- Tang, W.; Eisenbrand, G. Chinese Drugs of Plant Origin. Chemistry, Pharmacology, and Use in Traditional and Modern Medicine; Springer-Verlag: Berlin Heidelberg, Germany, 1992; pp. 545–547. [Google Scholar]
- Ding, C.S.; Shen, Y.S.; Li, G.; Wei, Z.; Wei, F. Study of a glycoprotein from Gastrodia elata: Its effects of anticoagulation and antithrombosis. Zhongguo Zhong Yao Za Zhi 2007, 32, 1060–1064. [Google Scholar]
- Gutiérrez, R.M.P. Orchids: A review of uses in traditional medicine, its phytochemistry and pharmacology. J. Med. Plants Res 2010, 4, 592–638. [Google Scholar]
- Hong, Q.M.; Shi, S.S.; Wang, C.; Wang, S.C.; Li, X.L.; Zhou, X.J. Structural characterization of a glucan isolated from Gastrodia elata. Zhong Yao Cai 2010, 33, 726–729. [Google Scholar]
- Tong, X.K.; Qiu, H.; Zhang, X.; Shi, L.P.; Wang, G.F.; Ji, F.H.; Ding, H.Y.; Tang, W.; Ding, K.; Zuo, J.P. WSS45, a sulfated alpha-D-glucan, strongly interferes with Dengue 2 virus infection in vitro. Acta Pharmacol. Sin 2010, 31, 585–592. [Google Scholar]
- Hayashi, J.; Sekine, T.; Deguchi, S.; Lin, Q.; Horie, S.; Tsuchiya, S.; Yano, S.; Watanabe, K.; Ikegami, F. Phenolic compounds from Gastrodia rhizome and relaxant effects of related compounds on isolated smooth muscle preparation. Phytochemistry 2002, 59, 513–539. [Google Scholar]
- Kim, H.J.; Moon, K.D.; Lee, D.S.; Lee, S.H. Ethyl ether fraction of Gastrodia elata Blume protects amyloid beta peptide-induced cell death. J. Ethnopharmacol 2003, 84, 95–98. [Google Scholar]
- Hong, H.D.; Kim, Y.C.; Keum, I.K.; Kim, S.S.; Kim, K.I.; Han, C.K. Effect of Gastrodia rhizome fractions on serum lipid concentrations in rats fed with high fat diet. J. Korean Soc. Appl. Biol. Chem 2005, 48, 370–374. [Google Scholar]
- Xu, X.; Lu, Y.; Bie, X. Protective effects of gastrodin on hypoxia-induced toxicity in primary cultures of rat cortical neurons. Planta Med 2007, 73, 650–654. [Google Scholar]
- Zhu, H.B.; Geng, M.Y.; Guan, H.S.; Zhang, J.T. Antihypertensive effects of d-polymannuronic sulfate and its related mechanisms in renovascular hypertensive rats. Acta Pharmacol. Sin 2000, 21, 727–732. [Google Scholar]
- Hou, W.C.; Lee, M.H.; Hsu, F.L.; Lin, Y.H. Inhibitory activities of semicarbazide-sensitive amine oxidase and angiotensin converting enzyme of pectin hydroxamic acid. J. Agric. Food Chem 2003, 51, 6362–6366. [Google Scholar]
- Matsui, T.; Zhu, X.L.; Shiraishi, K.; Ueki, T.; Noda, Y.; Matsumoto, K. Antihypertensive effect of salt-free soy sauce, a new fermented seasoning, in spontaneously hypertensive rats. J. Food Sci 2010, 75, H129–H134. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Dong, Q.; Fang, J.; Li, X. Colorimetric method for determination of sugars and related substance. Anal. Chem 1956, 28, 350–356. [Google Scholar]
- Thetsrimuang, C.; Khammuang, S.; Sarnthima, R. Antioxidant activity of crude polysaccharides from edible fresh and dry mushroom fruiting bodies of Lentinus sp. strain RJ-2. Int. J. Pharmacol 2011, 7, 58–65. [Google Scholar]
- Han, C.K.; Lee, O.H.; Kim, K.I.; Park, J.M.; Kim, Y.C.; Lee, B.Y. Effect of powder, 50% ethanol and hot water extracts of Gastrodiae Rhizoma on serum lipids and blood pressure in SHR fed high-fat diet. J. Korean Soc. Food Sci. Nutr 2003, 32, 1095–1101. [Google Scholar]
- Rhyu, M.R.; Kim, E.Y.; Han, J.S. Antihypertensive effect of the soybean paste fermented with the fungus Monascus. Int. J. Food Sci. Technol 2002, 37, 585–588. [Google Scholar]
- Lee, Y.K.; Woo, M.H.; Kim, C.H.; Kim, Y.; Lee, S.H.; Jeong, B.S.; Chang, H.W.; Son, J.K. Two new benzofurans from Gastrodia elata and their DNA topoisomerases I and II inhibitory activities. Planta Med 2007, 73, 1287–1291. [Google Scholar]
- Lee, B.Y.; Choi, H.S.; Hwang, J.B. Analysis of food components of Gratrodiae Rhizoma and changes in several characteristics at the various drying conditions. Korean J. Food Sci. Technol 2002, 34, 37–42. [Google Scholar]
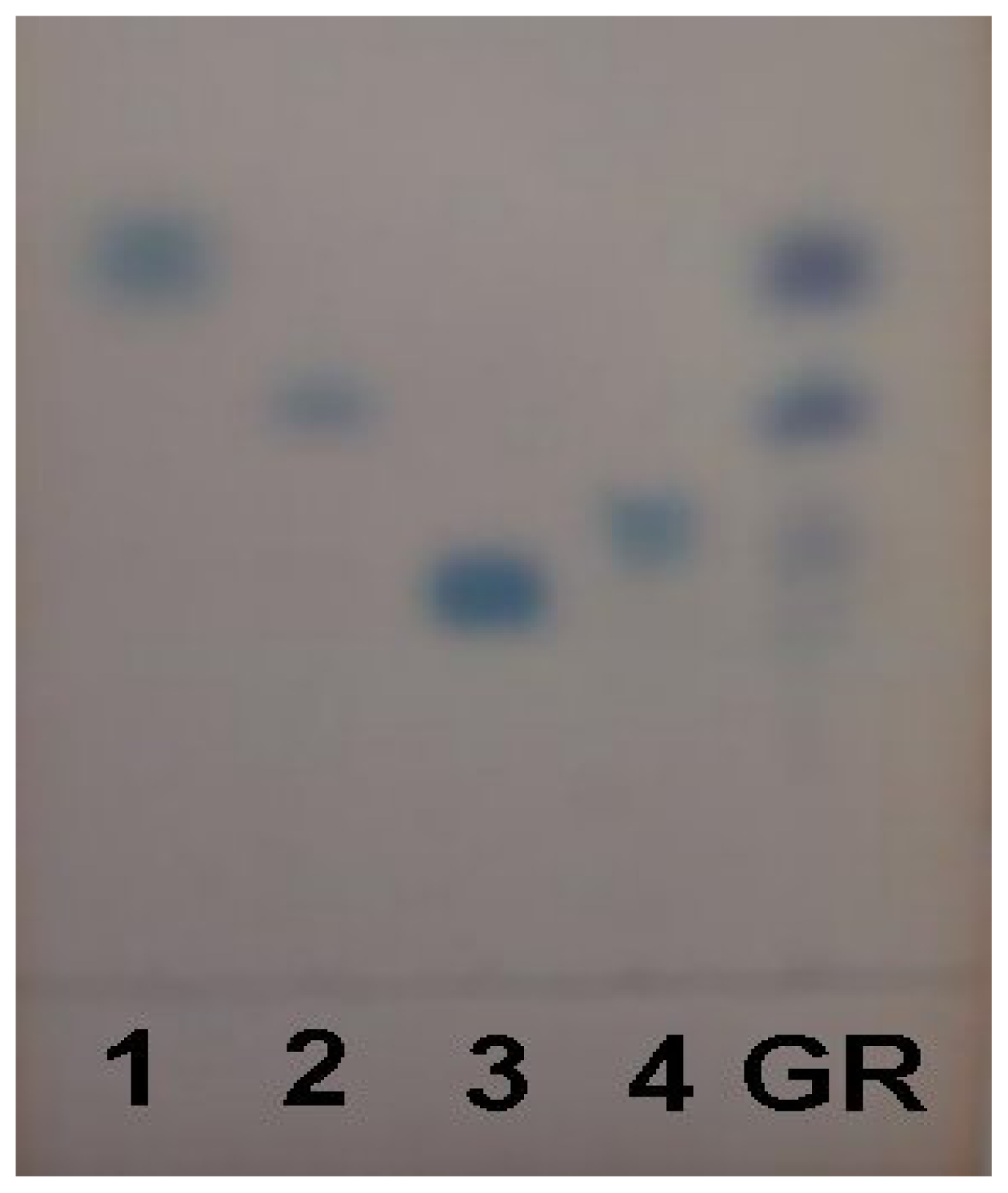

| Yield (g) | Total protein (%) | Total sugar (%) | Acidic polysaccharides (%) | |
|---|---|---|---|---|
| Crude polysaccharides fraction | 2.47 | 1.19 ± 0.11 | 86.25 ± 1.54 | 12.56 ± 1.15 |
| Acidic polysaccharide fraction | 0.61 | 0.32 ± 0.02 | 82.40 ± 1.16 | 17.28 ± 0.58 |
| Group (n = 12) | Lipids (mg/dL) and AI | ||||
|---|---|---|---|---|---|
| TC 1 | TG 2 | HDL 3 | LDL 4 | AI 5 | |
| A | 75.0 6 ± 6.0 b | 133.0 ± 9.4 b | 28.8 ± 1.6 b | 24.4 ± 1.8 b | 1.61 ± 0.31 b 7 |
| B | 69.7 ± 10.6 b | 131.0 ± 8.7 b | 31.0 ± 2.0 a | 22.2 ± 3.6 b | 1.24 ± 0.22 c |
| C | 89.2 ± 7.4 a | 166.7 ± 16.3 a | 27.0 ± 0.9 b | 28.6 ± 3.8 a | 2.31 ± 0.27 a |
| Ingredients | Content (%) |
|---|---|
| Casein (feed grade CP 85%) | 20.00 |
| Corn starch | 39.75 |
| Dextrinized corn starch | 13.20 |
| Sucrose | 10.00 |
| Soybean oil | 7.00 |
| Cellulose (fiber) | 5.00 |
| Mineral mixture 1 | 3.50 |
| Vitamin mixture 2 | 1.00 |
| l-Cystine | 0.30 |
| Choline bitartrate | 0.25 |
| Group (n = 12) | 1st phase (3 weeks) | 2nd phase (5 weeks) |
|---|---|---|
| A 1 | HFD 2 | Crude polysaccharides + HFD |
| B | HFD | Acidic polysaccharides + HFD |
| C | HFD | HFD |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, O.-H.; Kim, K.-I.; Han, C.-K.; Kim, Y.-C.; Hong, H.-D. Effects of Acidic Polysaccharides from Gastrodia Rhizome on Systolic Blood Pressure and Serum Lipid Concentrations in Spontaneously Hypertensive Rats Fed a High-Fat Diet. Int. J. Mol. Sci. 2012, 13, 698-709. https://doi.org/10.3390/ijms13010698
Lee O-H, Kim K-I, Han C-K, Kim Y-C, Hong H-D. Effects of Acidic Polysaccharides from Gastrodia Rhizome on Systolic Blood Pressure and Serum Lipid Concentrations in Spontaneously Hypertensive Rats Fed a High-Fat Diet. International Journal of Molecular Sciences. 2012; 13(1):698-709. https://doi.org/10.3390/ijms13010698
Chicago/Turabian StyleLee, Ok-Hwan, Kyung-Im Kim, Chan-Kyu Han, Young-Chan Kim, and Hee-Do Hong. 2012. "Effects of Acidic Polysaccharides from Gastrodia Rhizome on Systolic Blood Pressure and Serum Lipid Concentrations in Spontaneously Hypertensive Rats Fed a High-Fat Diet" International Journal of Molecular Sciences 13, no. 1: 698-709. https://doi.org/10.3390/ijms13010698
APA StyleLee, O.-H., Kim, K.-I., Han, C.-K., Kim, Y.-C., & Hong, H.-D. (2012). Effects of Acidic Polysaccharides from Gastrodia Rhizome on Systolic Blood Pressure and Serum Lipid Concentrations in Spontaneously Hypertensive Rats Fed a High-Fat Diet. International Journal of Molecular Sciences, 13(1), 698-709. https://doi.org/10.3390/ijms13010698






