Molecular Modeling Study of Chiral Separation and Recognition Mechanism of β-Adrenergic Antagonists by Capillary Electrophoresis
Abstract
:1. Introduction
2. Experimental
2.1. Apparatus
2.2. Chemicals
2.3. Preparation of BGE Solutions and Analytes
2.4. Molecular Construction and Optimization
2.5. Docking and Mechanic Calculations
2.6. Chromatographic Thermodynamics Calculations
3. Results and Discussion
3.1. Optimization of Chiral Separations
3.1.1. Effect of the Type of CD
3.1.2. Effect of Buffer pH
3.1.3. Effect of the CD Concentration
3.1.4. Effect of the Buffer Concentration, Voltage, and Temperature
3.1.5. Optimum Conditions for Enantioseparation
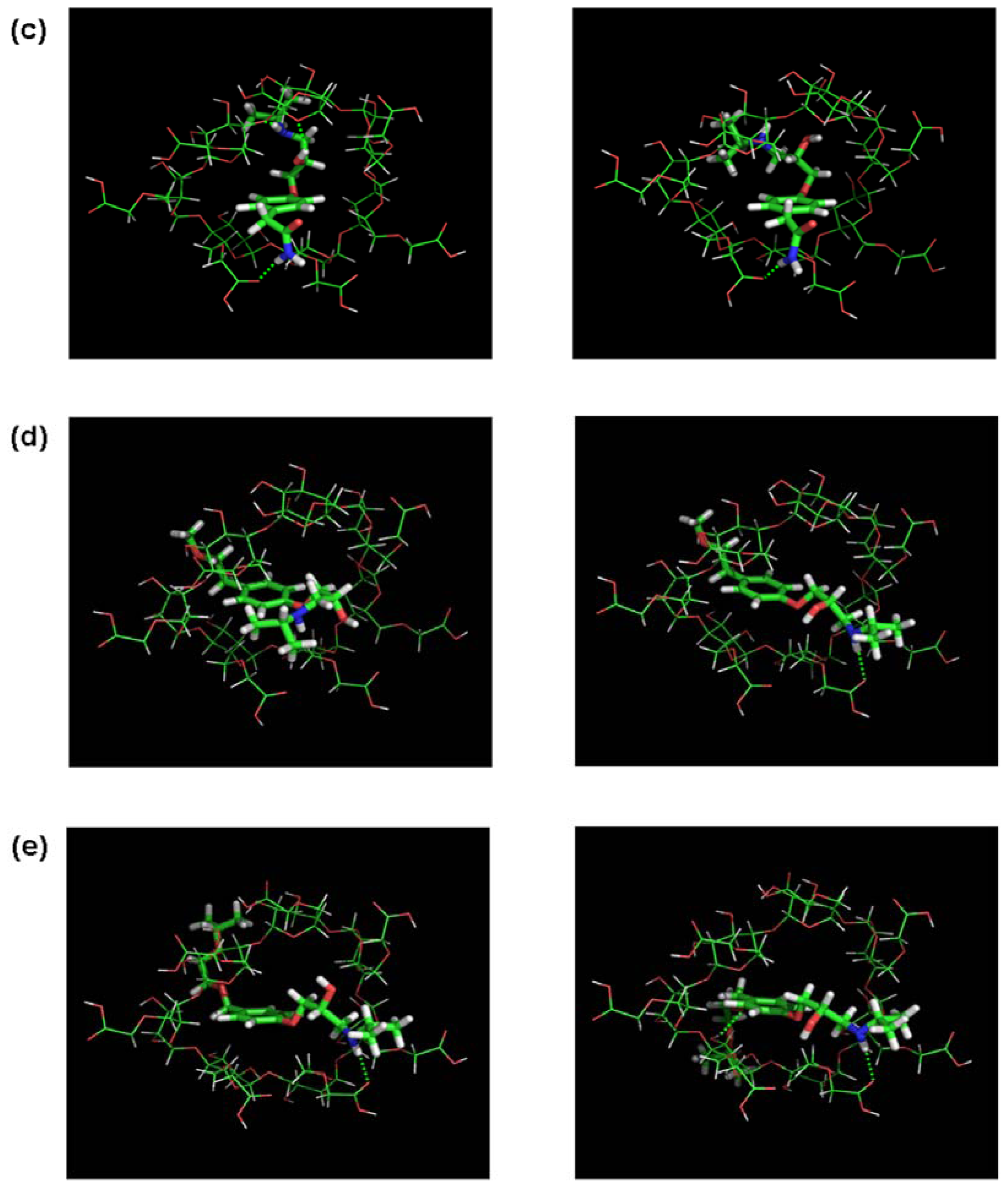
3.2. Molecular Docking and Chiral Recognition Mechanism
4. Conclusions
Acknowledgments
References
- McClure, D.E.; Baldwin, J.J.; Randall, W.C.; Lyon, T.F.; Mensler, K.; Lundell, G.F.; Raab, A.W.; Gross, D.; Risley, E.A.; Sweet, C.S.; et al. Antihypertensive β-adrenergic blocking agents: N-aralkyl analogues of 2-[3-(tert-butylamino)-2-hydroxypropoxy]-3-cyanopyridine. J. Med. Chem 1983, 26, 649–657. [Google Scholar]
- Barrett, A.M.; Cullum, V.A. Lack of inter-action between propranolol and mebanazine. J. Pharm. Pharmacol 1968, 20, 911–915. [Google Scholar]
- Barrett, A.M.; Cullum, V.A. The biological properties of the opticalisomers of propranolol and their effects on cardiacarrhythmias. Br. J. Pharmacol 1968, 34, 43–55. [Google Scholar]
- Bhushan, R.; Tanwar, S. Different approaches of impregnation for resolution of enantiomers of atenolol, propranolol and salbutamol using Cu(II)-l-amino acid complexes for ligand exchange on commercial thin layer chromatographic plates. J. Chromatogr. A 2010, 1217, 1395–1398. [Google Scholar]
- Bhushan, R.; Tanwar, S. Direct TLC resolution of atenolol and propranolol into their enantiomers using three different chiral selectors as impregnating reagents. Biomed. Chromatogr 2008, 22, 1028–1034. [Google Scholar]
- Bhushan, R.; Tanwar, S. Reversed-phase high-performance liquid chromatographic enantioresolution of six β-blockers using dinitrophenyl-l-Pro-N-hydroxysuccinimide ester, N-succinimidyl-(S)-2-(6- methoxynaphth-2-yl) propionate and twelve variants of Sanger’s reagent as chiral derivatizing reagents. Biomed. Chromatogr 2009, 23, 1291–1299. [Google Scholar]
- Ali, I.; Gaitonde, V.D.; Aboul-Enein, H.Y.; Hussain, A. Chiral separation of β-adrenergic blockers on CelluCoat column by HPLC. Talanta 2009, 78, 458–463. [Google Scholar]
- Younes, A.A.; Mangelings, D.; Heyden, Y.V. Chiral separations in normal phase liquid chromatography: Enantioselectivity of recently commercialized polysaccharide-based selectors. Part I: Enantioselectivity under generic screening conditions. J. Pharm. Biomed. Anal 2011, 55, 414–423. [Google Scholar]
- Kumar, A.P.; Park, J.H. Fast separations of chiral β-blockers on a cellulose tris(3,5- dimethylphenylcarbamate)-coated zirconia monolithic column by capillary electrochromatography. J. Chromatogr. A 2011, 1218, 5369–5373. [Google Scholar]
- Lu, M.; Zhang, L.; Qiu, B.; Feng, Q.; Xia, S.; Chen, G. Rapid separation and sensitive detection method for β-blockers by pressure-assisted capillary electrochromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2008, 1193, 156–163. [Google Scholar]
- Zhou, S.; Wang, Y.; de Beer, T.; Baeyens, W.R.G.; Fei, G.T.; Dilinuer, M.; Ouyang, J. Simultaneous separation of eight β-adrenergic drugs using titanium dioxide nanoparticles as additive in capillary electrophoresis. Electrophoresis 2008, 29, 2321–2329. [Google Scholar]
- Borges, K.B.; Pupo, M.T.; Bonato, P.S. Enantioselective analysis of propranolol and 4-hydroxypropranolol by CE with application to biotransformation studies employing endophytic fungi. Electrophoresis 2009, 30, 3910–3917. [Google Scholar]
- Borges, K.B.; Pupo, M.T.; de Freitas, L.A.P.; Bonato, P.S. Box-Behnken design for the optimization of an enantioselective method for the simultaneous analysis of propranolol and 4-hydroxypropranolol by CE. Electrophoresis 2009, 30, 2874–2881. [Google Scholar]
- Chen, J.; Du, Y.; Zhu, F.; Chen, B. Evaluation of the enantioselectivity of glycogen-based dual chiral selector systems towards basic drugs in capillary electrophoresis. J. Chromatogr. A 2010, 1217, 7158–7163. [Google Scholar]
- Blanco, M.; Valverde, I. Choice of chiral selector for enantioseparation by capillary electrophoresis. Trends Anal. Chem 2003, 22, 428–439. [Google Scholar]
- Park, K.-L.; Kim, K.H.; Jung, S.-H.; Lim, H.-M.; Hong, C.-H.; Kang, J.-S. Enantioselective stabilization of inclusion complexes of metoprolol in carboxymethylated β-cyclodextrin. J. Pharm. Biomed. Anal 2002, 27, 569–576. [Google Scholar]
- Servais, A.-C.; Rousseau, A.; Fillet, M.; Lomsadze, K.; Salgado, A.; Crommen, J.; Chankvetadze, B. Separation of propranolol enantiomers by CE using sulfated β-CD derivatives in aqueous and non-aqueous electrolytes: Comparative CE and NMR study. Electrophoresis 2010, 31, 1467–1474. [Google Scholar]
- Servais, A.-C.; Rousseau, A.; Fillet, M.; Lomsadze, K.; Salgado, A.; Crommen, J.; Chankvetadze, B. Capillary electrophoretic and nuclear magnetic resonance studies on the opposite affinity pattern of propranolol enantiomers towards various cyclodextrins. J. Sep. Sci 2010, 33, 1617–1624. [Google Scholar]
- Faucci, M.T.; Melani, F.; Mura, P. Computer-aided molecular modeling techniques for predicting the stability of drug-cyclodextrin inclusion complexes in aqueous solutions. Chem. Phys. Lett 2002, 358, 383–390. [Google Scholar]
- Elbashir, A.A.; Suliman, F.E.O. Computational modeling of capillary electrophoretic behavior of primary amines using dual system of 18-crown-6 and β-cyclodextrin. J. Chromatogr. A 2011, 1218, 5344–5351. [Google Scholar]
- Eid, E.E.M.; Abdul, A.B.; Suliman, F.E.O.; Sukari, M.A.; Rasedee, A.; Fatah, S.S. Characterization of the inclusion complex of zerumbone with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym 2011, 83, 1707–1714. [Google Scholar] [Green Version]
- Chakraborty, S.; Basu, S.; Lahiri, A.; Basak, S. Inclusion of chrysin in β-cyclodextrin nanocavity and its effect on antioxidant potential of chrysin: A spectroscopic and molecular modeling approach. J. Mol. Struct 2010, 977, 180–188. [Google Scholar]
- Jullian, C.; Alfaro, M.; Zapata-Torres, G.; Olea-Azar, C. Inclusion complexes of cyclodextrins with galangin: A thermodynamic and reactivity study. J. Solut. Chem 2010, 39, 1168–1177. [Google Scholar]
- Chaudhuri, S.; Chakraborty, S.; Sengupta, P.K. Encapsulation of serotonin in β-cyclodextrin nano-cavities: Fluorescence spectroscopic and molecular modeling studies. J. Mol. Struct 2010, 975, 160–165. [Google Scholar]
- Bikádi, Z.; Fodor, G.; Hazai, I.; Hári, P.; Szemán, J.; Szente, L.; Fülöp, F.; Péter, A.; Hazai, E. Molecular modeling of enantioseparation of phenylazetidin derivatives by cyclodextrins. Chromatographia 2010, 71, 21–28. [Google Scholar]
- Bednarek, E.; Bocian, W.; Michalska, K. NMR and molecular modeling study, as complementary techniques to capillary electrophoresis method to elucidate the separation mechanism of linezolid enantiomers. J. Chromatogr. A 2008, 1193, 164–171. [Google Scholar]
- Waibel, B.; Scheiber, J.; Meier, C.; Hammitzsch, M.; Baumann, K.; Scriba, G.K.E.; Holzgrabe, U. Comparison of cyclodextrin-dipeptide inclusion complexes in the absence and presence of urea by means of capillary electrophoresis, nuclear magnetic resonance and molecular modeling. Eur. J. Org. Chem 2007, 2007, 2921–2930. [Google Scholar]
- Kahle, C.; Deubner, R.; Schollmayer, C.; Scheiber, J.; Baumann, K.; Holzgrabe, U. NMR spectroscopic and molecular modelling studies on cyclodextrin-dipeptide inclusion complexes. Eur. J. Org. Chem 2005, 2005, 1578–1589. [Google Scholar]
- ChemStation software, version A.08.03; Hewlett-Packard: Palo Alto, CA, USA, 2001.
- SYBYL, version 7.0; Tripos Associates Inc.: St. Louis, MO, USA, 2004.
- Powell, M.J.D. An efficient method for finding the minimum of a function of several variables without calculating derivatives. Comput. J 1964, 7, 155–162. [Google Scholar]
- MOPAC, version 6.0; Indiana University: Bloomington, IN, USA, 1989.
- van Galen, P.J.; van Vlijmen, H.W.; AP, I.J.; Soudijn, W. A model for the antagonist binding site on the adenosine A1 receptor, based on steric, electrostatic, and hydrophobic properties. J. Med. Chem 1990, 33, 1708–1713. [Google Scholar]
- Ludwig, O.; Schinke, H.; Brandt, W. Reparametrisation of force constants in MOPAC 6.0/7.0 for better description of the activation barrier of peptide bond rotations. J. Mol. Model 1996, 2, 341–350. [Google Scholar]
- Banerjee, A.; Mikhailova, E.; Cheley, S.; Gu, L.Q.; Montoya, M.; Nagaoka, Y.; Gouaux, E.; Bayley, H. Molecular bases of cyclodextrin adapter interactions with engineered protein nanopores. Proc. Natl. Acad. Sci. USA 2010, 107, 8165–8170. [Google Scholar]
- Autodock, version 3.0; Scripps Research Institute: La Jolla, CA, USA, 1998.
- Osterberg, F.; Morris, G.M.; Sanner, M.F.; Olson, A.J.; Goodsell, D.S. Automated docking to multiple target structures: Incorporation of protein mobility and structural water heterogeneity in AutoDock. Proteins 2002, 46, 34–40. [Google Scholar]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem 1998, 19, 1639–1662. [Google Scholar]
- Penn, S.G.; Bergstrom, E.T.; Goodall, D.M.; Loran, J.S. Capillary electrophoresis with chiral selectors: Optimization of separation and determination of thermodynamic parameters for binding of tioconazole enantiomers to cyclodextrins. Anal. Chem 1994, 66, 2866–2873. [Google Scholar]
- Lelivre, F.; Gareil, P.; Jardy, A. Selectivity in capillary electrophoresis:application to chiral separations with cyclodextrins. Anal. Chem 1997, 69, 385–392. [Google Scholar]
- Morante-Zarcero, S.; Crego, A.L.; Sierra, I.; Fajardo, M.; Marina, M.L. Chiral capillary electrophoresis applied to the determination of phenylglycidol enantiomers obtained from cinnamyl alcohol by asymmetric epoxidation using new titanium(IV) alkoxide compounds as catalysts. Electrophoresis 2004, 25, 2745–2754. [Google Scholar]
- Wren, S.A.C.; Rowe, R.C. Theoretical aspects of chiral separation in capillary electrophoresis: I. Initial evaluation of a model. J. Chromatogr. A 1992, 603, 235–241. [Google Scholar]
- Wang, Z.; Ouyang, J.; Baeyens, W.R.G. Recent developments of enantioseparation techniques for adrenergic drugs using liquid chromatography and capillary electrophoresis: A review. J. Chromatogr. B 2008, 862, 1–14. [Google Scholar]
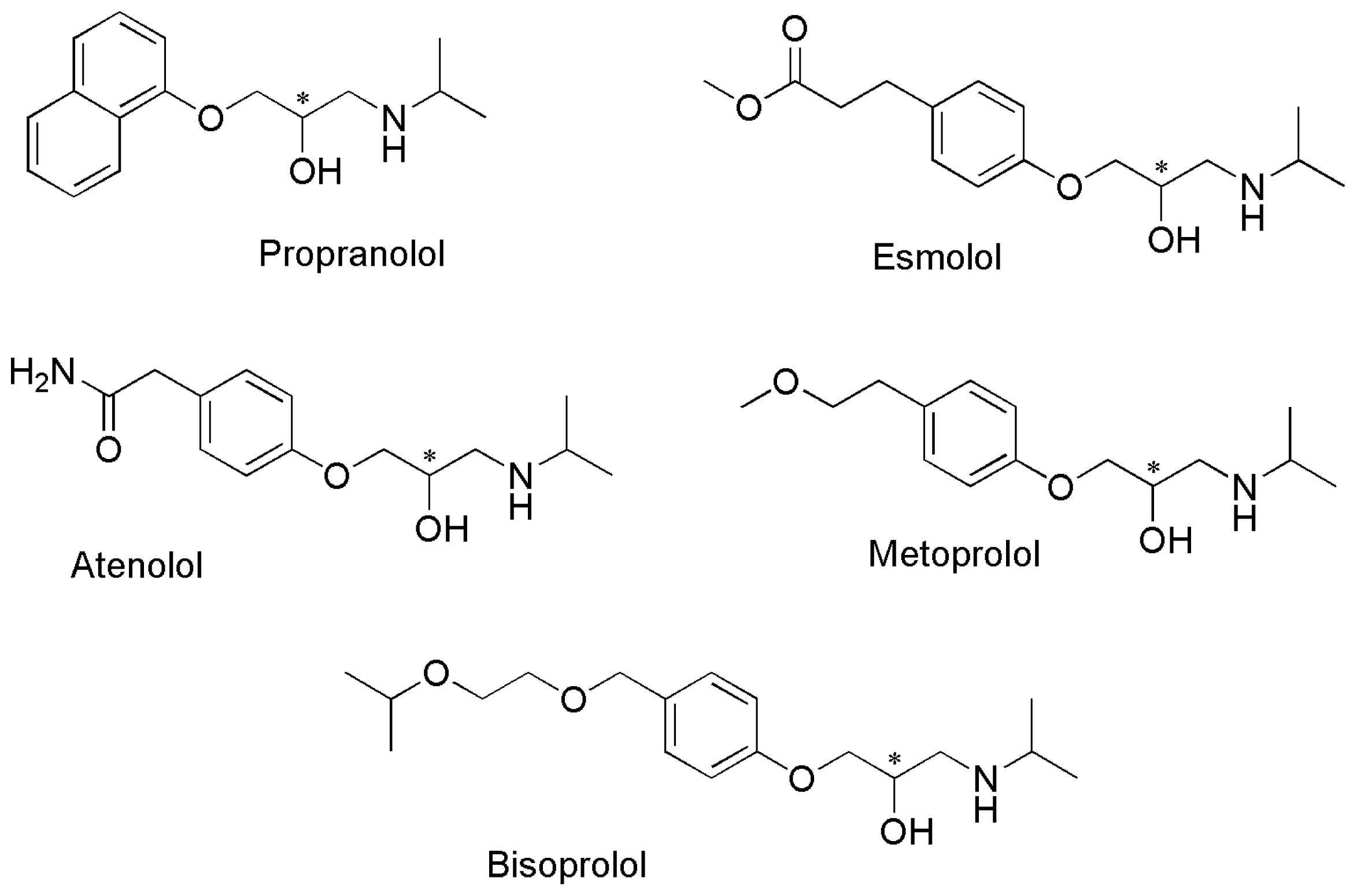
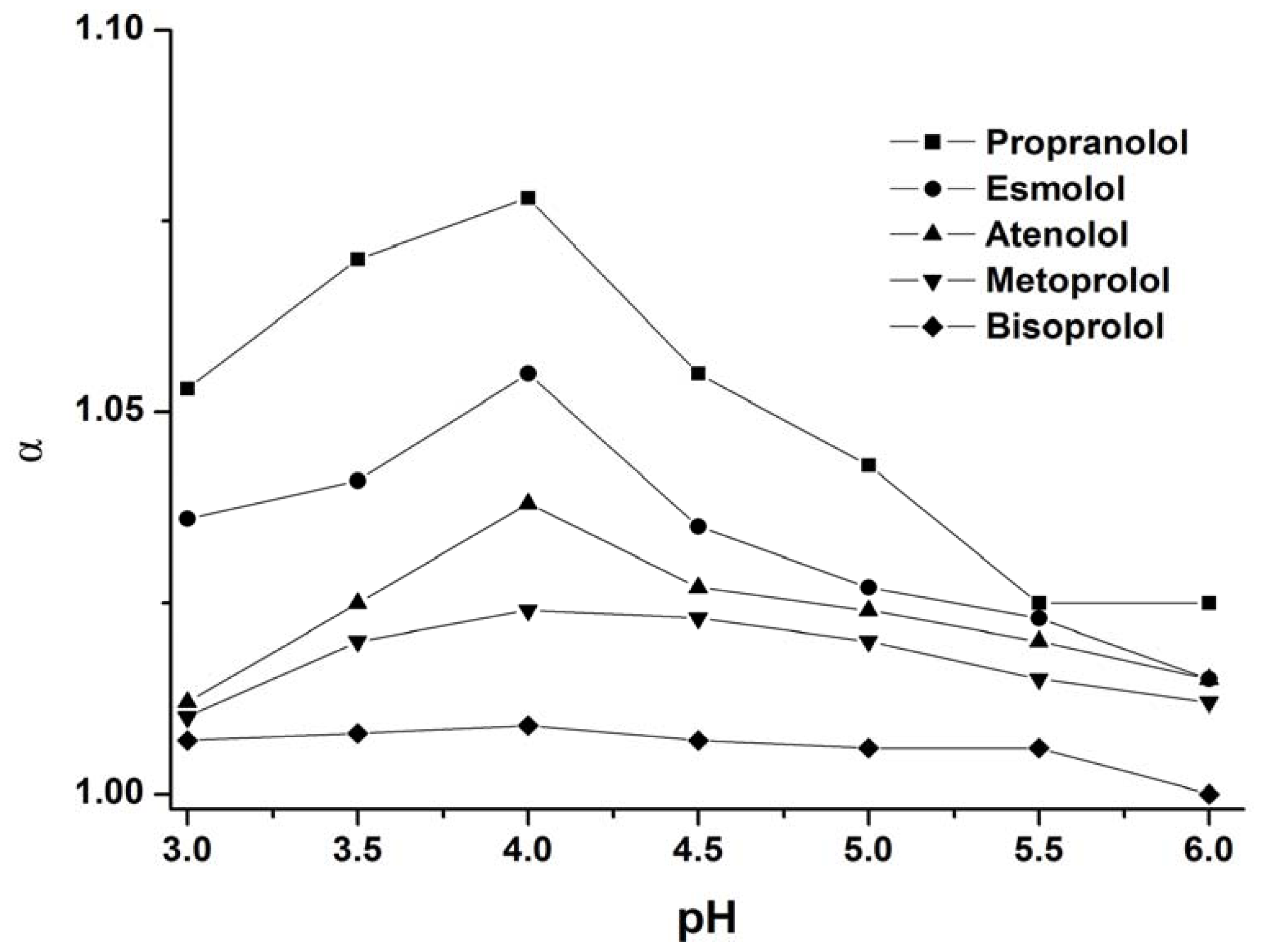

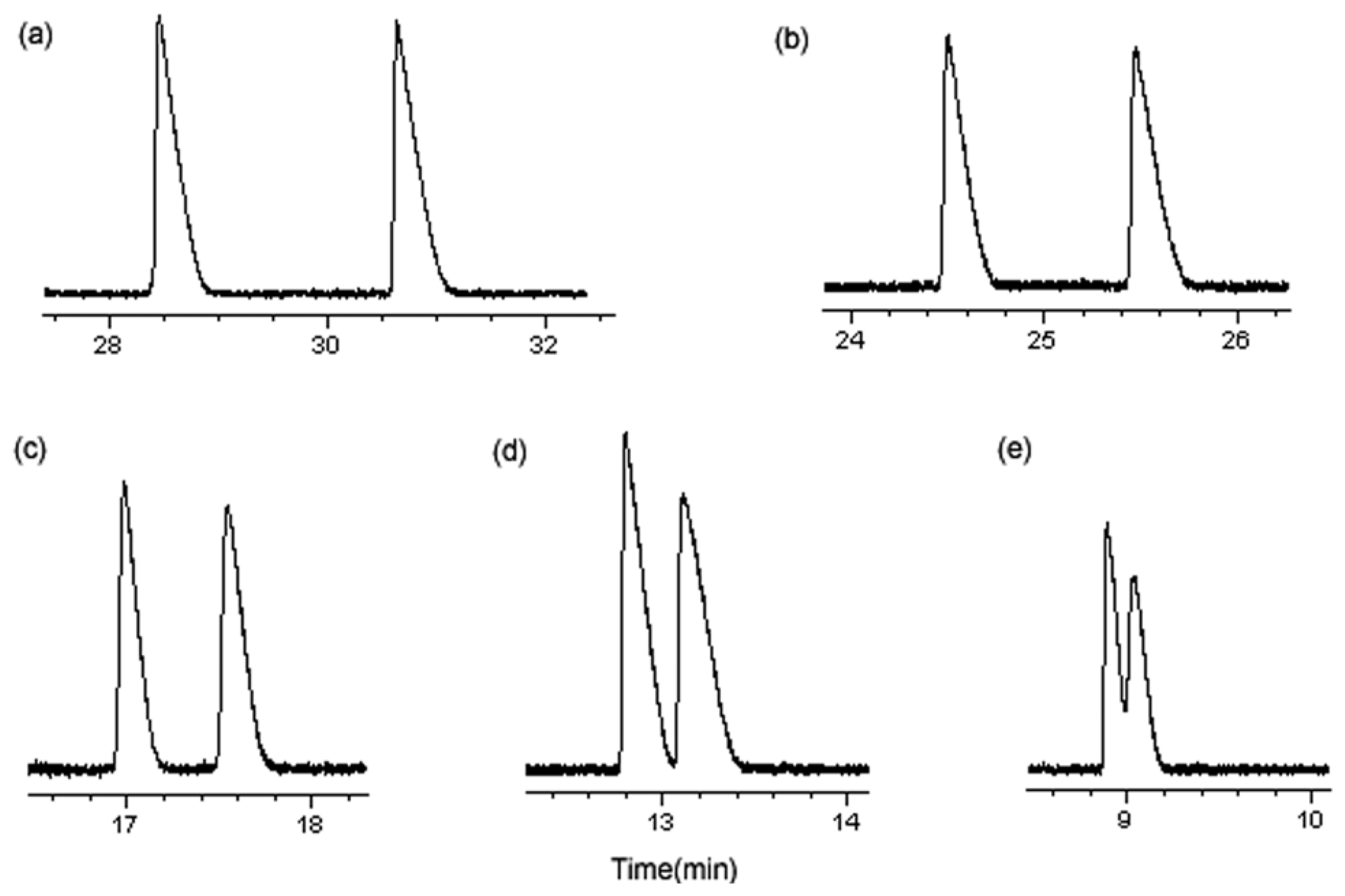
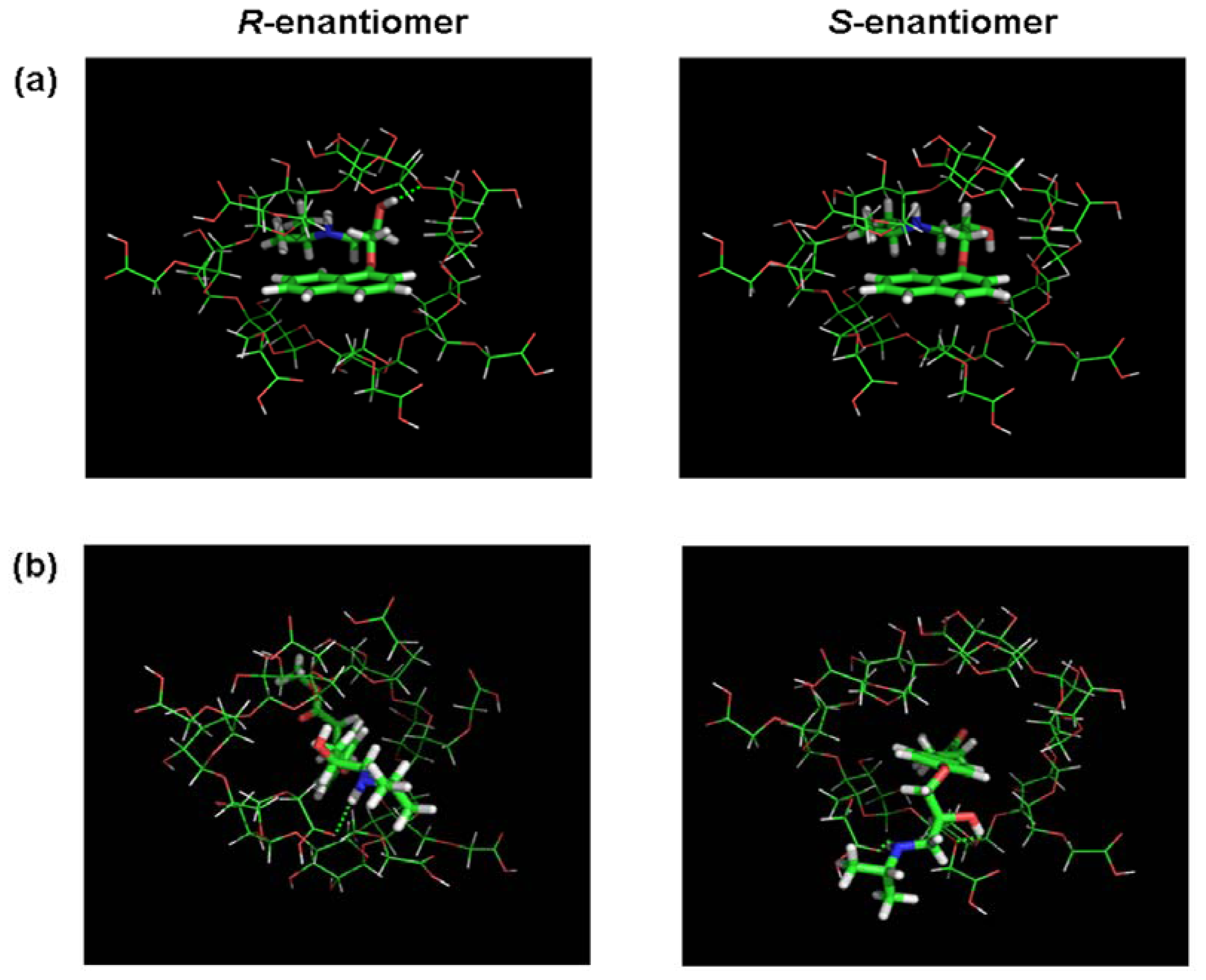

| Enantiomers | Selectivity Factors (α) | |||||
|---|---|---|---|---|---|---|
| β-CD | HP-β-CD | DM-β-CD | TM-β-CD | S-β-CD | CM-β-CD | |
| Propranolol | 1.009 | 1.016 | 1.010 | 1.015 | 1.019 | 1.053 |
| Esmolol | 1.000 | 1.012 | 1.007 | 1.010 | 1.006 | 1.036 |
| Atenolol | 1.000 | 1.006 | 1.000 | 1.000 | 1.007 | 1.012 |
| Metoprolol | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.010 |
| Bisoprolol | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.007 |
| Enantiomers | Binding Free Energy (ΔG) (kcal/mol) | Difference of Binding Free Energy (|ΔΔG|) (kcal/mol) | Selectivity Factors (α) ** | ||
|---|---|---|---|---|---|
| R-ΔG * | S-ΔG * | Calculated | Experimental | ||
| Propranolol | −4.340 | −3.926 | 0.414 | 0.183 | 1.078 |
| Esmolol | −5.113 | −5.469 | 0.356 | 0.131 | 1.055 |
| Atenolol | −5.984 | −5.679 | 0.305 | 0.091 | 1.038 |
| Metoprolol | −3.410 | −3.622 | 0.212 | 0.057 | 1.024 |
| Bisoprolol | −4.612 | −4.747 | 0.135 | 0.022 | 1.009 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, W.; Liu, C.; Tan, G.; Zhang, X.; Zhu, Z.; Chai, Y. Molecular Modeling Study of Chiral Separation and Recognition Mechanism of β-Adrenergic Antagonists by Capillary Electrophoresis. Int. J. Mol. Sci. 2012, 13, 710-725. https://doi.org/10.3390/ijms13010710
Li W, Liu C, Tan G, Zhang X, Zhu Z, Chai Y. Molecular Modeling Study of Chiral Separation and Recognition Mechanism of β-Adrenergic Antagonists by Capillary Electrophoresis. International Journal of Molecular Sciences. 2012; 13(1):710-725. https://doi.org/10.3390/ijms13010710
Chicago/Turabian StyleLi, Wuhong, Changhai Liu, Guangguo Tan, Xinrong Zhang, Zhenyu Zhu, and Yifeng Chai. 2012. "Molecular Modeling Study of Chiral Separation and Recognition Mechanism of β-Adrenergic Antagonists by Capillary Electrophoresis" International Journal of Molecular Sciences 13, no. 1: 710-725. https://doi.org/10.3390/ijms13010710




