Expression, Immobilization and Enzymatic Properties of Glutamate Decarboxylase Fused to a Cellulose-Binding Domain
Abstract
:1. Introduction
2. Results and Discussion
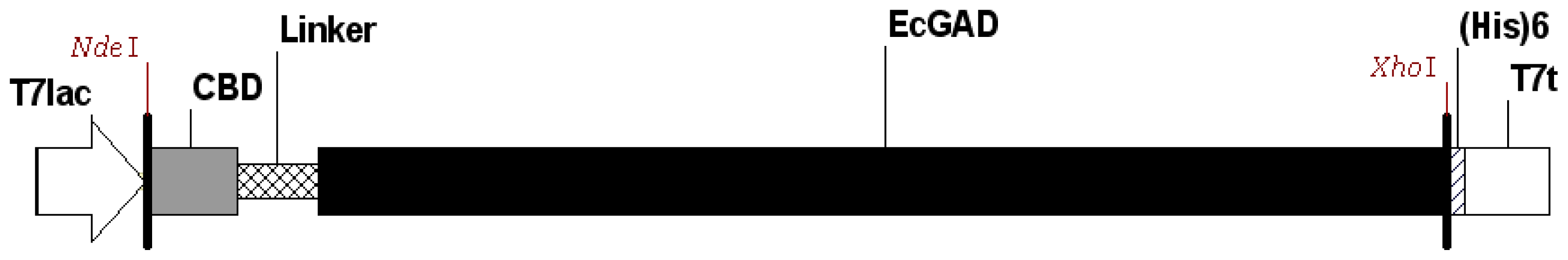
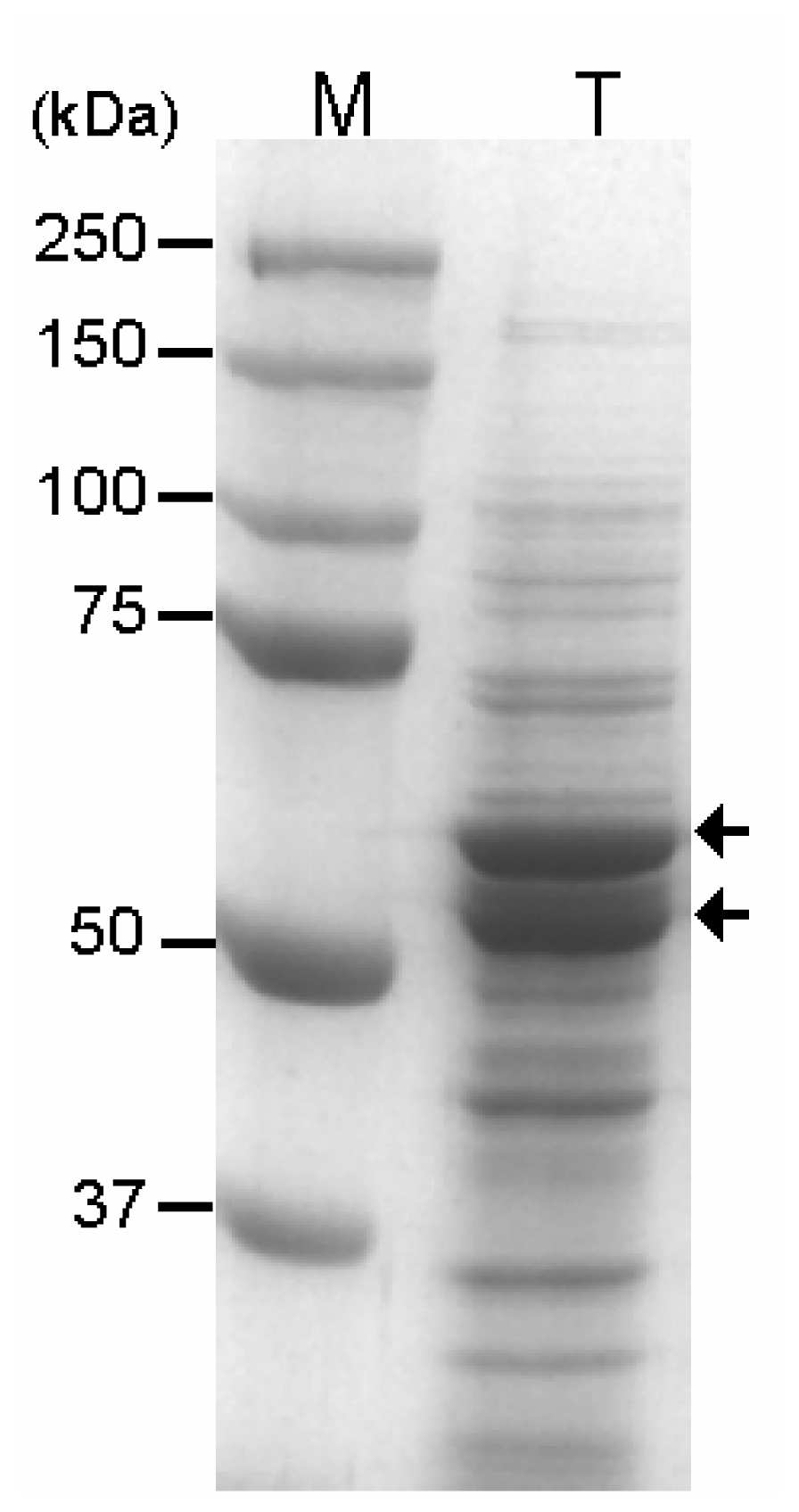
2.1. Expression of GAD Fused to CBD in E. coli
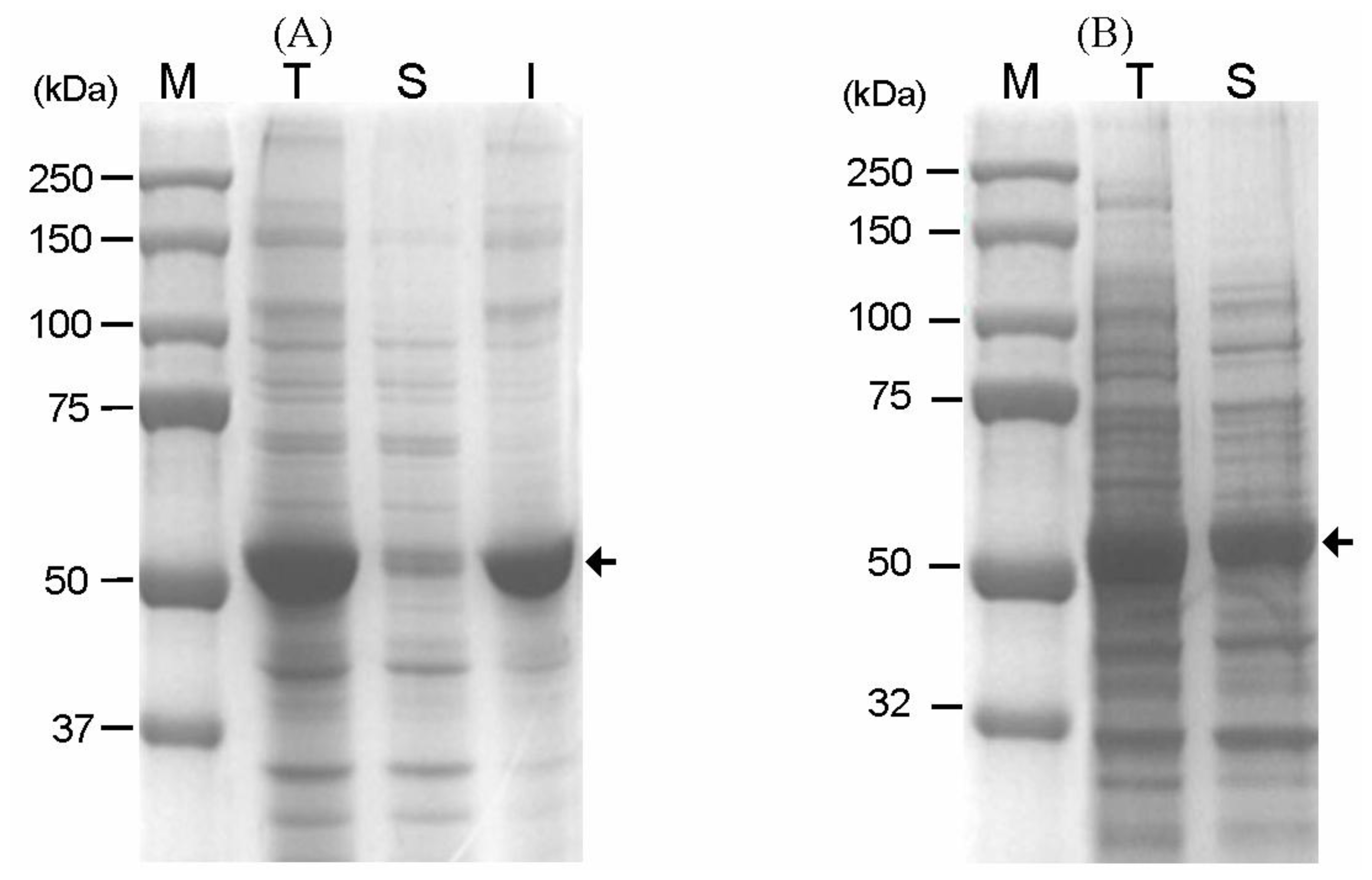
2.2. Modification of the Linker Peptide Connecting CBD with GAD
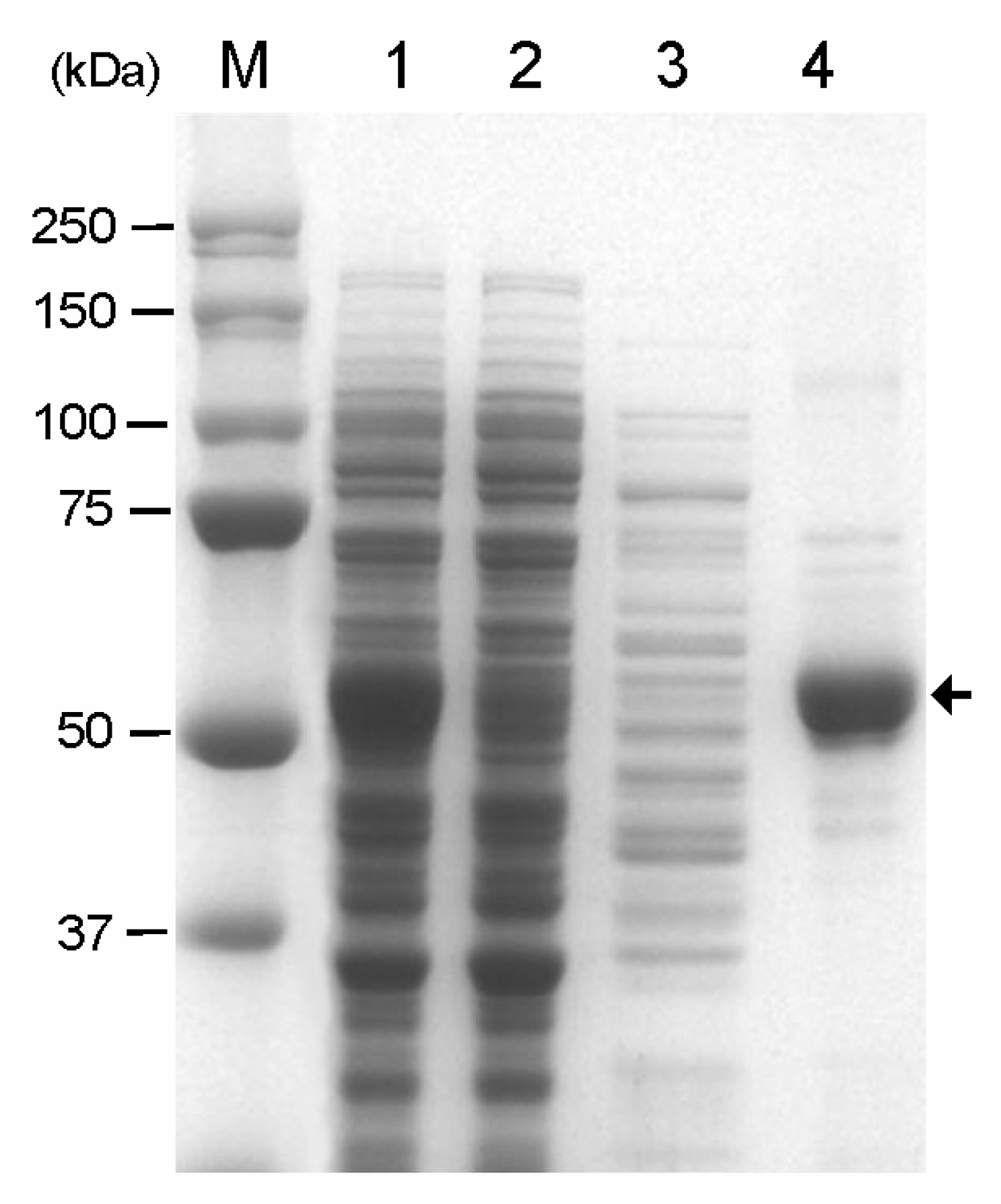
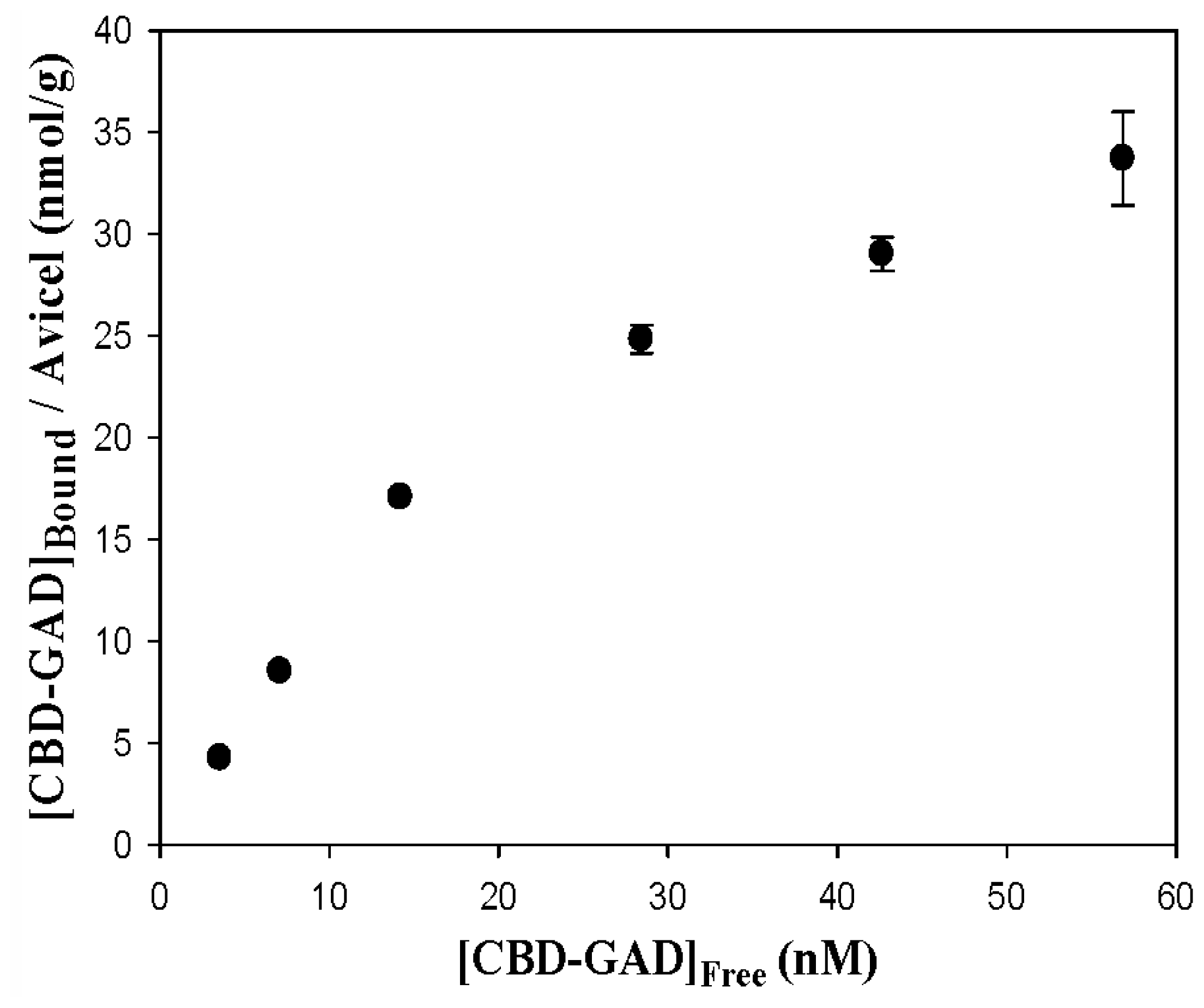
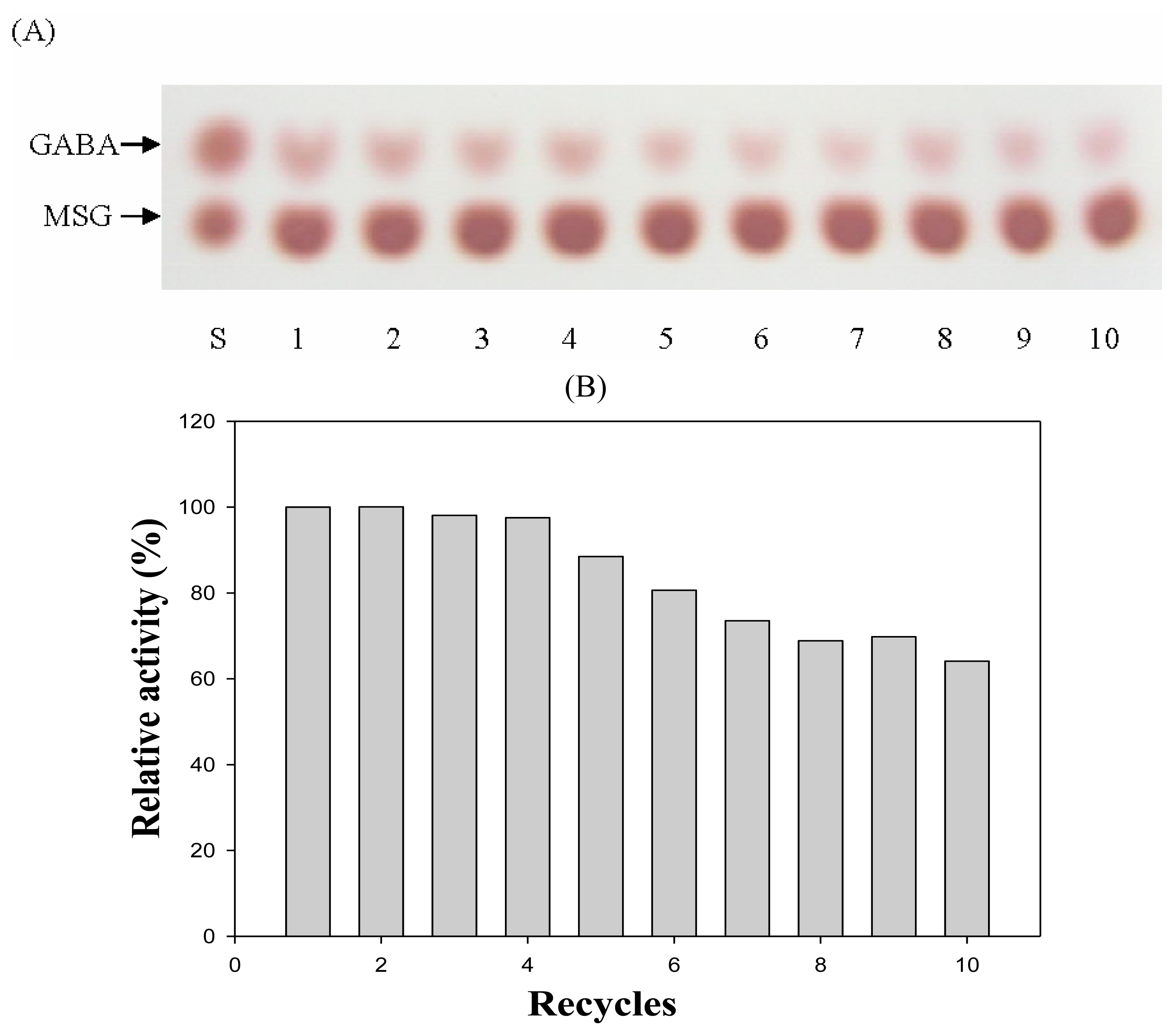
2.3. Immobilization of CBD-GAD to the Cellulose Matrix
3. Experimental Section
3.1. Bacterial Srains
3.2. Plasmid Construction
3.3. Expression of CBD-GAD
3.4. N-Terminal Amino Acid Sequencing
3.5. Purification of CBD-GAD and GAD Protein
3.6. Assay of GAD Activity
3.7. Adsorption Isotherm Measurements
3.8. Reusability Assay of Immobilized GAD
4. Conclusions
Acknowledgments
References
- Ueno, Y.; Hayakawa, K.; Takahashi, S.; Oda, K. Purification and characterization of glutamate decarboxylase from lactobacillus brevis IFO 12005. Biosci. Biotechnol. Biochem 1997, 61, 1168–1171. [Google Scholar]
- Fenalti, G.; Law, R.H.P.; Buckle, A.M.; Langendorf, C.; Tuck, K.; Rosado, C.J.; Faux, N.G.; Mahmood, K.; Hampe, C.S.; Banga, J.P.; et al. GABA production by glutamic acid decarboxylase is regulated by a dynamic catalytic loop. Nat. Struct. Mol. Biol 2007, 14, 280–286. [Google Scholar]
- Erlander, M.G.; Tobin, A.J. The structural and functional heterogeneity of glutamic acid decarboxylase: A review. Neurochem. Res 1991, 16, 215–226. [Google Scholar]
- Hiraga, K.; Ueno, Y.; Oda, K. Glutamate decarboxylase from Lactobacillus brevis: Activation by ammonium sulfate. Biosci. Biotechnol. Biochem 2008, 72, 1299–1306. [Google Scholar]
- Foster, J.W. Escherichia coli acid resistance: Tales of an amateur acidophile. Nat. Rev. Microbiol 2004, 2, 898–907. [Google Scholar]
- Pennacchietti, E.; Lammens, T.M.; Capitani, G.; Franssen, M.C.R.; John, R.A.; Bossa, F.; De Biase, D. Mutation of His465 alters the pH-dependent spectroscopic properties of Escherichia coli glutamate decarboxylase and broadens the range of its activity toward more alkaline pH. J. Biol. Chem 2009, 284, 31587–31596. [Google Scholar]
- Lammens, T.M.; De Biase, D.; Franssen, M.C.R.; Scott, E.L.; Sanders, J.P.M. The application of glutamic acid α-decarboxylase for the valorization of glutamic acid. Green Chem 2009, 11, 1562–1567. [Google Scholar]
- Jung, I.L.; Kim, I.G. Polyamines and glutamate decarboxylase-based acid resistance in Escherichia coli. J. Biol. Chem 2003, 278, 22846–22852. [Google Scholar]
- Plokhov, A.Y.; Gusyatiner, M.M.; Yampolskaya, T.A.; Kaluzhsky, V.E.; Sukhareva, B.S.; Schulga, A.A. Preparation of γ-aminobutyric acid using E. coli Cells with high activity of glutamate decarboxylase. Appl. Biochem. Biotechnol. Part A 2000, 88, 257–265. [Google Scholar]
- De Biase, D.; Tramonti, A.; John, R.A.; Bossa, F. Isolation, overexpression, and biochemical characterization of the two isoforms of glutamic acid decarboxylase from Escherichia coli. Protein Expr. Purif 1996, 8, 430–438. [Google Scholar]
- Capitani, G.; De Biase, D.; Aurizi, C.; Gut, H.; Bossa, F.; Grütter, M.G. Crystal structure and functional analysis of Escherichia coli glutamate decarboxylase. EMBO J 2003, 22, 4027–4037. [Google Scholar]
- Kavoosi, M.; Creagh, A.L.; Kilburn, D.G.; Haynes, C.A. Strategy for selecting and characterizing linker peptides for CBM9-tagged fusion proteins expressed in Escherichia coli. Biotechnol. Bioeng 2007, 98, 599–610. [Google Scholar]
- Gilkes, N.R.; Jervis, E.; Henrissat, B.; Tekant, B.; Miller, R.C., Jr; Warren, R.A.J.; Kilburn, D. G. The adsorption of a bacterial cellulase and its two isolated domains to crystalline cellulose. J. Biol. Chem 1992, 267, 6743–6749. [Google Scholar]
- Goldstein, M.A.; Takagi, M.; Hashida, S.; Shoseyov, O.; Doi, R.H.; Segel, I.H. Characterization of the cellulose-binding domain of the Clostridium cellulovorans cellulose-binding protein A. J. Bacteriol 1993, 175, 5762–5768. [Google Scholar]
- Hall, M.; Bansal, P.; Lee, J.H.; Realff, M.J.; Bommarius, A.S. Biological pretreatment of cellulose: Enhancing enzymatic hydrolysis rate using cellulose-binding domains from cellulases. Bioresour. Technol 2011, 102, 2910–2915. [Google Scholar]
- De Los Ángeles Calixto-Romo, M.; Santiago-Hernández, J.A.; Vallejo-Becerra, V.; Amaya-Delgado, L.; Del Carmen Montes-Horcasitas, M.; Hidalgo-Lara, M.E. Expression, purification and immobilization of the intracellular invertase INVA, from Zymomonas mobilis on crystalline cellulose and Nylon-6. J. Ind. Microbiol. Biotechnol 2008, 35, 1455–1463. [Google Scholar]
- Ito, S.; Kuno, A.; Suzuki, R.; Kaneko, S.; Kawabata, Y.; Kusakabe, I.; Hasegawa, T. Rational affinity purification of native Streptomyces family 10 xylanase. J. Biotechnol 2004, 110, 137–142. [Google Scholar]
- Liu, Z.; Bartlow, P.; Dilmore, R.M.; Soong, Y.; Pan, Z.; Koepsel, R.; Ataai, M. Production, purification, and characterization of a fusion protein of carbonic anhydrase from Neisseria gonorrhoeae and cellulose binding domain from Clostridium thermocellum. Biotechnol. Prog 2009, 25, 68–74. [Google Scholar]
- Ahn, J.O.; Choi, E.S.; Lee, H.W.; Hwang, S.H.; Kim, C.S.; Jang, H.W.; Haam, S.J.; Jung, J.K. Enhanced secretion of Bacillus stearothermophilus L1 lipase in Saccharomyces cerevisiae by translational fusion to cellulose-binding domain. Appl. Microbiol. Biotechnol 2004, 64, 833–839. [Google Scholar]
- Santiago-Hernández, J.A.; Vásquez-Bahena, J.M.; Calixto-Romo, M.A.; Xoconostle-Cázares, G.B.; Ortega-López, J.; Ruíz-Medrano, R.; Montes-Horcasitas, M.C.; Hidalgo-Lara, M.E. Direct immobilization of a recombinant invertase to Avicel by E. coli overexpression of a fusion protein containing the extracellular invertase from Zymomonas mobilis and the carbohydrate-binding domain CBDCex from Cellulomonas fimi. Enzyme Microb. Technol 2006, 40, 172–176. [Google Scholar]
- Medve, J. Isotherms for adsorption of cellobiohydrolase 1 and II from trichoderma reesei on microcrystalline cellulose. Appl. Biochem. Biotechnol. Part A 1997, 66, 39–56. [Google Scholar]
- Tomme, P.; Heriban, V.; Claeyssens, M. Adsorption of two cellobiohydrolases from Trichoderma reesei to Avicel: Evidence for “exo-exo” synergism and possible “loose complex” formation. Biotechnol. Lett 1990, 12, 525–530. [Google Scholar]
- Laemmli, U.K.; Beguin, F.; Gujer-Kellenberger, G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J. Mol. Biol 1970, 47, 69–74. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Park, H.; Ahn, J.; Lee, J.; Lee, H.; Kim, C.; Jung, J.-K.; Lee, H.; Lee, E.G. Expression, Immobilization and Enzymatic Properties of Glutamate Decarboxylase Fused to a Cellulose-Binding Domain. Int. J. Mol. Sci. 2012, 13, 358-368. https://doi.org/10.3390/ijms13010358
Park H, Ahn J, Lee J, Lee H, Kim C, Jung J-K, Lee H, Lee EG. Expression, Immobilization and Enzymatic Properties of Glutamate Decarboxylase Fused to a Cellulose-Binding Domain. International Journal of Molecular Sciences. 2012; 13(1):358-368. https://doi.org/10.3390/ijms13010358
Chicago/Turabian StylePark, Hyemin, Jungoh Ahn, Juwhan Lee, Hyeokwon Lee, Chunsuk Kim, Joon-Ki Jung, Hongweon Lee, and Eun Gyo Lee. 2012. "Expression, Immobilization and Enzymatic Properties of Glutamate Decarboxylase Fused to a Cellulose-Binding Domain" International Journal of Molecular Sciences 13, no. 1: 358-368. https://doi.org/10.3390/ijms13010358
APA StylePark, H., Ahn, J., Lee, J., Lee, H., Kim, C., Jung, J.-K., Lee, H., & Lee, E. G. (2012). Expression, Immobilization and Enzymatic Properties of Glutamate Decarboxylase Fused to a Cellulose-Binding Domain. International Journal of Molecular Sciences, 13(1), 358-368. https://doi.org/10.3390/ijms13010358



