Role of α-Helical Structure in Organic Solvent-Activated Homodimer of Elastase Strain K
Abstract
:1. Introduction
2. Results and Discussion
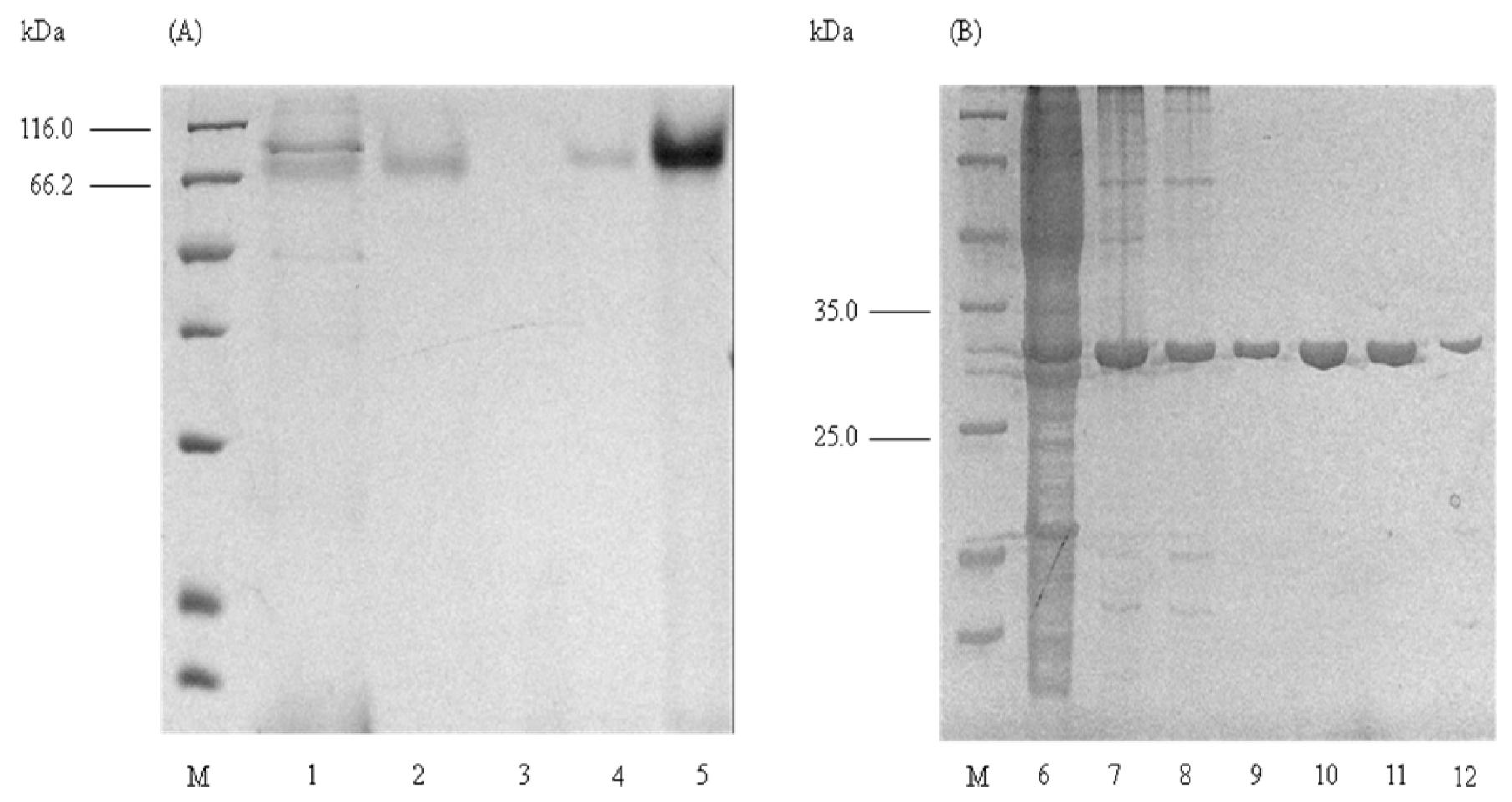
2.1. Purification of Elastase Strain K
2.2. Determination of Molecular Mass
2.3. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight/Time-of-Flight (MALDI ToF/ToF) Mass Spectrometry
2.4. Native PAGE and Activity Staining
2.5. Characterization of Elastase Strain K
2.5.1. Effect of Temperatures on Enzyme Activity and Stability
2.5.2. Effect of pH on Enzyme Activity and Stability
2.5.3. Effect of Additional Metal Ions on Enzyme Stability
2.5.4. Effect of Inhibitors on Enzyme Stability
2.5.5. Effect of Denaturing and Reducing Agents on Enzyme Stability
2.5.6. Substrate Specificity
2.5.7. Effect of Organic Solvents on Enzyme Stability
2.5.8. Effect of Methanol on Enzyme Activity and Structure
3. Experimental Section
3.1. Preparation of Crude Elastase Strain K for Protein Purification
3.2. Hydrophobic Interaction Chromatography (HIC)
3.3. Ion Exchange Chromatography (IEX)
3.4. Characterization of Recombinant Elastase Strain K
3.4.1. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight/Time-of-Flight (MALDI ToF/ToF)
3.4.2. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.4.3. Activity Staining
3.4.4. Effect of Temperature on Elastinolytic Activity
3.4.5. Effect of pH on Elastinolytic Activity
3.4.6. Effect of Additional Metal Ions on Elastinolytic Activity
3.4.7. Effect of Protease Inhibitors on Elastinolytic Activity
3.4.8. Effect of Denaturing and Reducing Agents on Elastinolytic Activity
3.4.9. Substrate Specificity of Recombinant Elastase Strain K
3.4.10. Organic Solvent Stability of Recombinant Elastase Strain K
3.4.11. Effect of Methanol Concentrations on Enzyme Stability
3.4.12. Biophysical Characterization of Recombinant Elastase Strain K
3.4.13. Effect of Methanol on Protein Secondary Structure
4. Conclusions
Supplementary Information
ijms-12-05797-s001.pdfAcknowledgements
References
- Krieger, N; Bhatnagar, T; Baratti, JC; Baron, AM; de Lima, VM; Mitchell, D. Non-aqueous biocatalysis in heterogeneous solvent systems. Food Technol Biotechnol 2004, 42, 279–286. [Google Scholar]
- Ogino, H; Watanabe, F; Yamada, M; Nakagawa, S; Hirose, T; Noguchi, A; Yasuda, M; Ishikawa, H. Purification and characterization of organic solvent-stable protease from organic solvent-tolerant Pseudomonas aeruginosa PST-01. J Biosci Bioeng 1999, 87, 61–68. [Google Scholar]
- Karadzic, I; Masui, A; Fujiwara, N. Purification and characterization of a protease from Pseudomonas aeruginosa grown in cutting oil. J Biosci Bioeng 2004, 98, 145–152. [Google Scholar]
- Doddapaneni, KK; Tatineni, R; Vellanki, RN; Rachcha, S; Anabrolu, N; Narakuti, V; Mangamoori, LN. Purification and characterization of a solvent and detergent-stable novel protease from Bacillus cereus. Microbiol Res 2009, 164, 383–390. [Google Scholar]
- Rai, SK; Mukherjee, AK. Statistical optimization of production, purification and industrial application of a laundry detergent and organic solvent-stable subtilisin-like serine protease (Alzwiprase) from Bacillus subtilis DM-04. Biochem Eng J 2010, 48, 173–180. [Google Scholar]
- Ogino, H; Yasui, K; Shiotani, T; Ishihara, T; Ishikawa, H. Organic solvent stable-tolerant bacterium which secretes an organic solvent-stable proteolytic enzyme. Appl Environ Microbiol 1995, 61, 4258–4262. [Google Scholar]
- Torres, S; Castro, GR. Non-aqueous biocatalysis in homogeneous solvent systems. Food Technol Biotechnol 2004, 42, 271–277. [Google Scholar]
- Menaa, B; Menaa, F; Aiolfi-Guimaraes, C; Sharts, O. Silica-based nanoporous sol-gel glasses: from bioencapsulation to protein folding studies. Int J Nanotechnol 2010, 7, 1–45. [Google Scholar]
- Menaa, B; Montoneri, C; Menaa, F; Montoneri, E; Boffa, V; Sharts, O. Protein helical structure enhancement in biocompatible fluoro-phosphonate-based nanoporous silica glasses assessed by circular dichroism spectroscopy. Int J Nanotechnol 2011, 8, 471–491. [Google Scholar]
- Geok, LP; Razak, CNA; Rahman, RNZRA; Basri, M; Salleh, AB. Isolation and screening of an extracellular organic solvent-tolerant protease producer. Biochem Eng J 2003, 13, 73–77. [Google Scholar]
- Rahman, RNZRA; Geok, LP; Basri, M; Salleh, AB. Physical factors affecting the production of organic solvent-tolerant protease by Pseudomonas aeruginosa strain K. Bioresour Technol 2005, 96, 429–436. [Google Scholar]
- Rahman, RNZRA; Geok, LP; Basri, M; Salleh, AB. An organic solvent-tolerant protease from Pseudomonas aeruginosa strain K: nutritional factors affecting protease production. Enzyme Microb Technol 2005, 36, 749–757. [Google Scholar]
- Rahman, RNZRA; Geok, LP; Basri, M; Salleh, AB. An organic solvent-stable alkaline protease from Pseudomonas aeruginosa strain K: Enzyme purification and characterization. Enzyme Microb Technol 2006, 39, 1484–1491. [Google Scholar]
- Yusoff, N. Purification and Characterization of Organic Solvent Tolerant Protease from Pseudomonas aeruginosa Strain K. Master Thesis, Universiti Putra Malaysia, Serdang, Malaysia, 2007. [Google Scholar]
- Xindu, G; Lili, W. Liquid chromatography of recombinant proteins and protein drugs. J Chromatogr B 2008, 866, 133–153. [Google Scholar]
- Geng, X; Wang, C. Protein folding liquid chromatography and its recent developments. J Chromatogr B 2007, 849, 69–80. [Google Scholar]
- Cheng, M; Takenaka, S; Aoki, S; Murakami, S; Aoki, K. Purification and characterization of an eggshell membrane decomposing protease from Pseudomonas aeruginosa strain ME-4. J Biosci Bioeng 2009, 107, 373–378. [Google Scholar]
- Lin, X; Xu, W; Huang, K; Mei, X; Liang, Z; Li, Z; Guo, J; Luo, Y. Cloning, expression and characterization of recombinant elastase from Pseudomonas aeruginosa in Pichia pastoris. Protein Expr Purif 2009, 63, 69–74. [Google Scholar]
- Li, ZR; Liu, GR; Cheng, Y. Thermodynamic analysis of protein sequence-structure relationships in monomer and dimer forms. Physica A 2005, 354, 381–392. [Google Scholar]
- Jones, S; Thornton, JM. Protein-protein interactions: a review of protein dimer structures. Prog Biophys Mol Biol 1995, 63, 31–65. [Google Scholar]
- McKee, T; JMcKee, JR. Enzymes; McGraw-Hill Higher Education: New York, NY, USA, 2003; pp. 161–199. [Google Scholar]
- Thayer, MM; Flaherty, KM; McKay, DB. Three-dimensional structure of elastase of Pseudomonas aeruginosa at 1.5-Å resolution. J Biol Chem 1991, 5, 2864–2871. [Google Scholar]
- Michalski, WP; Shiell, BJ. Strategies for analysis of electrophoretically separated proteins and peptides. Anal Chim Acta 1999, 383, 27–46. [Google Scholar]
- Schiffer, CA; Dötsch, V. The role of protein-solvent interactions in protein unfolding. Curr Opin Biotechnol 1996, 7, 428–432. [Google Scholar]
- Aldercreutz, P. Koskinen, AMP, Klibanov, AM, Eds.; Modes of using enzymes in organic media. In Enzymatic Reactions in Organic Media; Chapman and Hall: London, UK, 1996; pp. 9–37. [Google Scholar]
- Laane, C; Boeren, S; Vos, K; Veeger, C. Rules for optimization of biocatalysis in organic solvents. Biotechnol Bioeng 1987, 30, 81–87. [Google Scholar]
- Ru, MT; Dordick, JS; Reimer, JA; Clark, DS. Optimizing the salt-induced activation of enzymes in organic solvents: Effects of lyophilization time and water content. Biotechnol Bioeng 1999, 63, 233–241. [Google Scholar]
- Castro, GR. Enzymatic activities of proteases dissolved in organic solvents. Enzyme Microb Technol 1999, 25, 689–694. [Google Scholar]
- Knubovets, T; Osterhout, JJ; Klibanov, AM. Strucutres of lysozyme dissolved in neat organic solvents as assessed by NMR and CD spectroscopies. Biotechnol Bioeng 1999, 63, 242–248. [Google Scholar]
- Xu, K; Griebenow, K; Klibanov, AM. Correlation between catalytic activity and secondary structure of subtilisin dissolved in organic solvents. Biotechnol Bioeng 1997, 56, 485–491. [Google Scholar]
- Ogino, H; Gemba, Y; Yutori, Y; Doukyu, N; Ishimi, K; Ishikawa, H. Stabilities and conformational transitions of various proteases in the presence of an organic solvent. Biotechnol Prog 2007, 23, 155–161. [Google Scholar]
- Sharma, S; Tyagi, R; Gupta, MN; Singh, TP. Enhancement of catalytic activity of enzymes by heating in anhydrous organic solvents: 3D structure of a modified serine proteinase at high resolution. Indian J Biochem Biophys 2001, 38, 34–41. [Google Scholar]
- Ogino, H; Uchiho, T; Yokoo, J; Kobayashi, R; Ichise, R; Ishikawa, H. Role of intermolecular disulfide bonds of the organic solvent-stable PST-01 protease in its organic solvent stability. Appl Environ Microbiol 2001, 67, 942–947. [Google Scholar]
- Ogino, H; Uchiho, T; Doukyu, N; Yasuda, M; Ishimi, K; Ishikawa, H. Effect of exchange of amino acid residues of the surface region of the PST-01 protease on its organic solvent-stability. Biochem Biophys Res 2007, 358, 1028–1033. [Google Scholar]
- Castro, GR; Knubovets, T. Homogeneous biocatalysis in organic solvents and water-organic mixtures. Crit Rev Biotechnol 2003, 23, 195–231. [Google Scholar]
- Affleck, R; Haynes, CA; Clark, DS. Solvent dielectric effects on protein dynamics. Proc Natl Acad Sci USA 1992, 89, 5167–5170. [Google Scholar]
- Sreerama, N; Venyaminov, SY; Woody, RW. Estimation of the number of α-helical and β-strand segments in proteins using circular dichroism spectroscopy. Protein Sci 1999, 8, 370–380. [Google Scholar]
- Ohman, DE; Cryz, SJ; Iglewski, BH. Isolation and characterization of a Pseudomonas aerugionsa PAO mutant that produces altered elastase. J Bacteriol 1980, 142, 836–842. [Google Scholar]
- Laemmli, UK. Cleavage of structure protein during assembly of the head of bacteriophage T4. Nature 1970, 277, 680–685. [Google Scholar]
- Bollag, DM; Edelstein, SJ. Concentrating Protein Solutions; Wiley-Liss: New York, NY, USA, 1991. [Google Scholar]
- Cowan, DA; Smolenski, KA; Daniel, RM; Mogan, HW. An extremely thermostable extracellular proteinase from a strain of the archaebacterium Desulfurococcus growing at 88 °C. Biochem J 1987, 247, 121–133. [Google Scholar]
- Whitmore, L; Wallace, BA. DICROWEB: an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res 2004, 32, 668–673. [Google Scholar]
- Whitmore, L; Wallace, BA. Protein secondary structure analyses from circular dichroisn spectroscopy: methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar]



| Purification step | Volume (mL) | Activity (A495/h/mL) | Total activity (A495/h) | Protein content (mg/mL) | Total protein (mg) | Specific activity (A495/h/mg) | Yield (%) | Fold |
|---|---|---|---|---|---|---|---|---|
| Crude | 50 | 7 | 368 | 0.5 | 23 | 16 | 100 | 1 |
| HIC | 22 | 15 | 326 | 0.1 | 2 | 181 | 89 | 11 |
| IEX | 6.6 | 27 | 177 | 0.1 | 0.4 | 403 | 48 | 25 |
| Characterization | Characteristic | |
|---|---|---|
| Optimum temperature (°C) | 40 | |
| Thermal stability (°C) | 4–60 | |
| Optimum pH | 6 | |
| pH stability | 5–11 | |
| Relative activity (%) | ||
| 5.0 mM | 10.0 mM | |
| Control a | 100 | 100 |
| Metal ion | ||
| Na+ | 94 | 99 |
| K+ | 94 | 94 |
| Mg2+ | 94 | 89 |
| Ca2+ | 90 | 91 |
| Mn2+ | 90 | 93 |
| Co2+ | 90 | 87 |
| Ni2+ | 17 | 12 |
| Cu2+ | 81 | 55 |
| Zn2+ | 30 | 1 |
| Sr2+ | 92 | 94 |
| Fe3+ | 82 | 7 |
| Protease inhibitor | ||
| PMSF | 104 | 96 |
| EDTA | 32 | 5 |
| o-phenanthroline | 4 | 0 |
| Pepstatin A | 103 | 92 |
| Antipain b | 102 | 95 |
| Reducing and denaturing agent c | ||
| B-mercaptoethanol | 83 | |
| Triton-X-100 | 122 | |
| Tween 20 | 105 | |
| Urea (6 M) | 83 | |
| SDS | 12 | |
| DTT | 1 | |
| Substrate specificity | Casein, azocasein, elastin Congo-red, haemoglobin, egg albumin and Azocoll | |
| Concentration (% (v/v)) | Relative activity (%) a |
|---|---|
| 0 | 100 ± 4.52 |
| 25 | 115 ± 2.47 |
| 50 | 98 ± 1.44 |
| 75 | 30 ± 1.49 |
| 90 | 4 ± 0.13 |
| Methanol (% (v/v)) | α-helix a | α-helix b | β-sheet a | β-sheet b | Turn | Unordered |
|---|---|---|---|---|---|---|
| 0 | 0.19 | 0.12 | 0.19 | 0.10 | 0.20 | 0.20 |
| 25 | 0.15 | 0.11 | 0.20 | 0.10 | 0.20 | 0.24 |
| 50 | 0.10 | 0.09 | 0.22 | 0.11 | 0.19 | 0.30 |
| 75 | 0.02 | 0.06 | 0.22 | 0.12 | 0.20 | 0.38 |
| 90 | 0.00 | 0.06 | 0.21 | 0.11 | 0.19 | 0.42 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abd. Rahman, R.N.Z.R.; Salleh, A.B.; Basri, M.; Wong, C.F. Role of α-Helical Structure in Organic Solvent-Activated Homodimer of Elastase Strain K. Int. J. Mol. Sci. 2011, 12, 5797-5814. https://doi.org/10.3390/ijms12095797
Abd. Rahman RNZR, Salleh AB, Basri M, Wong CF. Role of α-Helical Structure in Organic Solvent-Activated Homodimer of Elastase Strain K. International Journal of Molecular Sciences. 2011; 12(9):5797-5814. https://doi.org/10.3390/ijms12095797
Chicago/Turabian StyleAbd. Rahman, Raja Noor Zaliha Raja, Abu Bakar Salleh, Mahiran Basri, and Chee Fah Wong. 2011. "Role of α-Helical Structure in Organic Solvent-Activated Homodimer of Elastase Strain K" International Journal of Molecular Sciences 12, no. 9: 5797-5814. https://doi.org/10.3390/ijms12095797
APA StyleAbd. Rahman, R. N. Z. R., Salleh, A. B., Basri, M., & Wong, C. F. (2011). Role of α-Helical Structure in Organic Solvent-Activated Homodimer of Elastase Strain K. International Journal of Molecular Sciences, 12(9), 5797-5814. https://doi.org/10.3390/ijms12095797




