Removal of Mercury by Foam Fractionation Using Surfactin, a Biosurfactant
Abstract
:1. Introduction
2. Results and Discussion
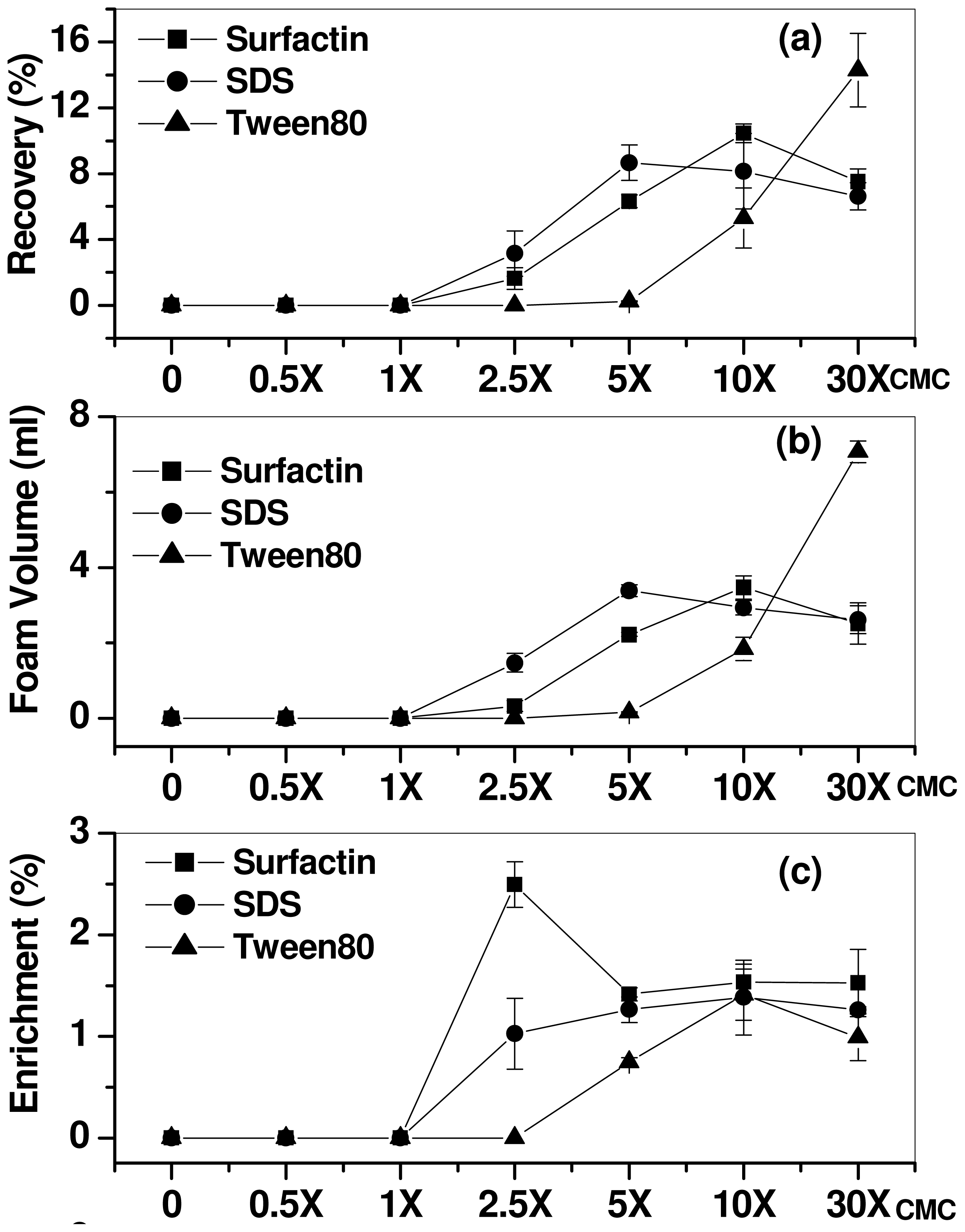
2.1. Effect of Surfactant Concentration
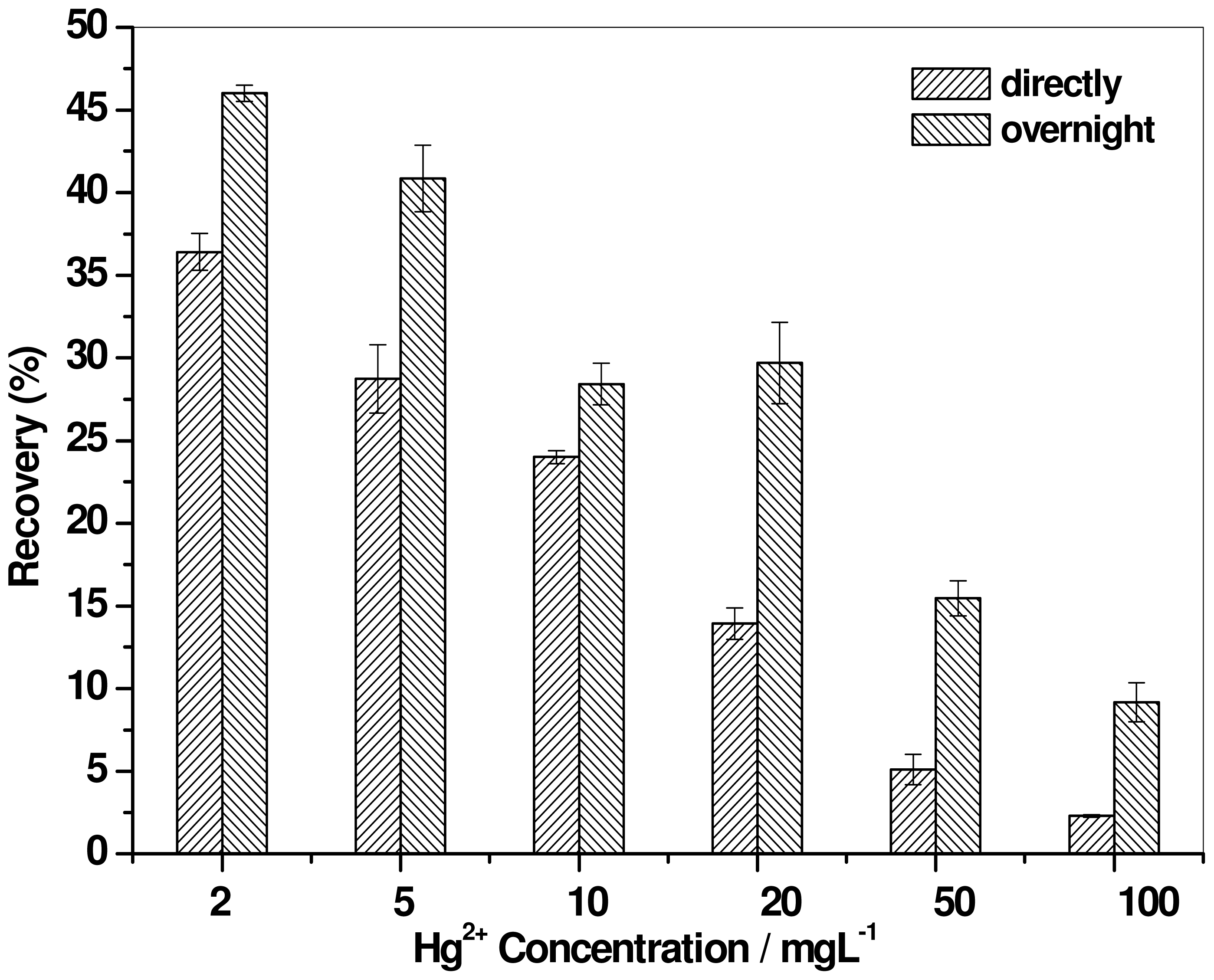
2.2. Effect of Mercury Concentration
2.3. Foaming Time Course
2.4. Effect of Digestion Time
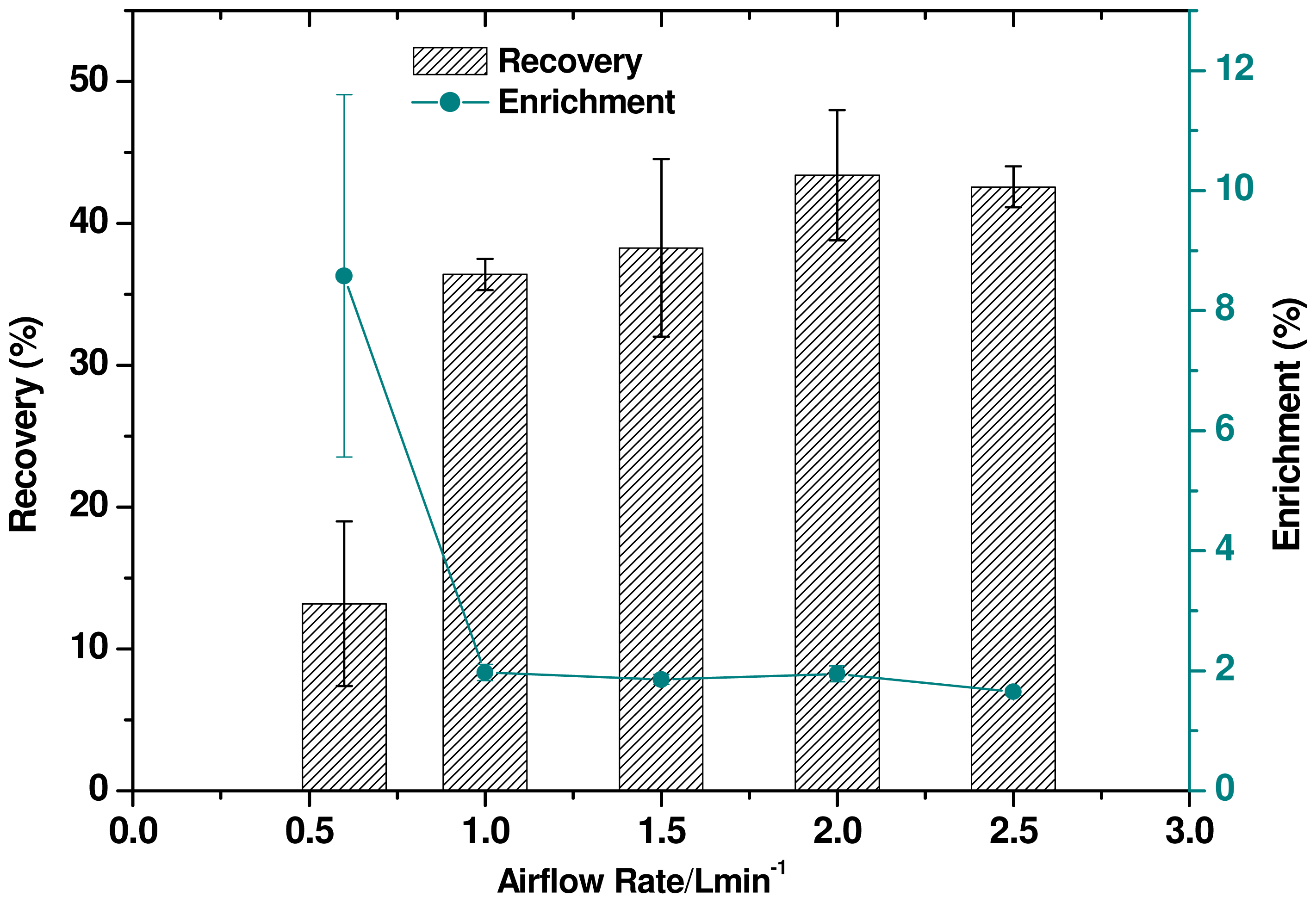
2.5. Effect of Airflow Rate
2.6. Effect of pH
3. Experimental Section
3.1. Surfactin Production
3.2. Experimental Set Up
3.3. Mercury Concentration Analysis
4. Conclusions
Acknowledgements
References
- Prokofev, A.K. Chemical forms of mercury, cadmium, and zinc in natural waters. Russ. Chem. Rev 1981, 50, 32–48. [Google Scholar]
- Hamdy, M.K.; Noyes, O.R. Formation of methyl mercury by bacteria. Appl. Environ. Microbiol 1975, 30, 424–432. [Google Scholar]
- Toxicological Effects of Methylmercury; Committee report for National Research Council; National Academies Press: Washington, DC, USA, 2000.
- Han, S.; Gill, G.A. Determination of mercury complexation in coastal and estuarine waters using competitive ligand exchange method. Environ. Sci. Technol 2005, 39, 6607–6615. [Google Scholar]
- Jay, J.A.; Morel, F.M.M.; Hemond, H.F. Mercury speciation in the presence of polysulfides. Environ. Sci. Technol 2000, 34, 2196–2200. [Google Scholar]
- Lee, T.H.; Jiang, S.J. Determination of mercury compounds by capillary electrophoresis inductively coupled plasma mass spectrometry with microconcentric nebulization. Anal. Chim. Acta 2000, 413, 197–205. [Google Scholar]
- Lee, S.H.; Park, Y.O. Gas-phase mercury removal by carbon-based sorbents. Fuel Process. Technol 2003, 84, 197–206. [Google Scholar]
- Dutta, S.; Bhattacharyyaa, A.; De, P.; Ray, P.; Basu, S. Removal of mercury from its aqueous solution using charcoal-immobilized papain (CIP). J. Hazard. Mater 2009, 172, 888–896. [Google Scholar]
- Melamed, R.; DaLuz, A.B. Efficiency of industrial minerals on the removal of mercury species from liquid effluents. Sci. Total Environ 2006, 368, 403– 406. [Google Scholar]
- Misra, M.; Lorengo, J.A. Method of Removing Mercury from Solution. US Patent 5599515, 4 February 1997. [Google Scholar]
- Barron-Zambrano, J.; Laborie, S.; Viers, P.; Rakib, M.; Durand, G. Mercury removal and recovery from aqueous solutions by coupled complexation-ultrafiltration and electrolysis. J. Membr. Sci 2004, 229, 179–186. [Google Scholar]
- Zeroual, Y.; Moutaouakkil, A.; Dzairi, F.Z.; Talbi, M.; Chung, P.U.; Lee, K.; Blaghen, M. Biosorption of mercury from aqueous solution by Ulva lactuca biomass. Bioresour. Technol 2003, 90, 349–351. [Google Scholar]
- Barron-Zambrano, J.; Laborie, S.; Viers, P.; Rakib, M.; Durand, G. Mercury removal from aqueous solutions by complexation-ultrafiltration. Desalination 2002, 144, 201–206. [Google Scholar]
- Reddy, K.R.; Parapudi, U.S.; Devulapalli, S.N.; Xu, C.Y. Effects of soil composition on the removal of chromium by electrokinetics. J. Hazard. Mater 1997, 55, 135–158. [Google Scholar]
- Mulligan, C.N.; Wang, S. Remediation of a heavy metal-contaminated soil by rhamnolipid foam. Eng. Geol 2006, 85, 75–81. [Google Scholar]
- Wang, S.; Mulligan, C.N. Rhamnolipid foam enhanced remediation of cadmium and nickel contaminated soil. Water Air Soil Pollut 2004, 157, 315–330. [Google Scholar]
- Baek, K.; Kim, B.K.; Cho, H.J.; Yang, J.W. Removal characteristics of anionic metals by micellar enhanced ultrafiltration. J. Hazard. Mater 2003, 99, 303–311. [Google Scholar]
- Ferella, F.; Prisciandaro, M.; Michelis, I.; Veglio, F. Removal of heavy metals by surfactant-enhanced ultrafiltration from wastewaters. Desalination 2007, 207, 125–133. [Google Scholar]
- De Castro Dantas, T.N.; Dantas Neto, A.A.; de Moura, A.M.C.P.; Barros Neto, E.L.; de Paiva Telemaco, E. Chromium adsorption by chitosan impregnated with microemulsion. Langmuir 2001, 17, 4256–4260. [Google Scholar]
- Darton, R.C.; Supino, S.; Sweeting, K.J. Development of a multistaged foam fractionation column. Chem. Eng. Proc 2004, 43, 477–482. [Google Scholar]
- Jafvert, C.T. Surfactants/Cosolvents; Technology Evaluation Report TE 96-02; Ground-Water Remediation Technologies Analysis Center (GWRTAC): Pittsburgh, PA, USA, 1996. [Google Scholar]
- Melek, D.Y. Recovery of Zn(II), Mn(II) and Cu(II) in aqueous solutions by foam fractionation with sodium dodecyl sulfate in combination with chelating agents. Sep. Sci. Technol 2006, 41, 1741–1756. [Google Scholar]
- Yuan, X.Z.; Meng, Y.T.; Zeng, G.M.; Fang, Y.Y.; Shi, J.G. Evaluation of tea-derived biosurfactant on removing heavy metal ions from dilute wastewater by ion floatation. Colloids Surf. A Physicochem. Eng. Asp 2008, 317, 256–261. [Google Scholar]
- Moussavi, M.; Javidnejad, M. Separation of Hg(II) by foam fractionation in the acidic range: Effect of complexation. J. Hazard. Mater 2007, 144, 187–193. [Google Scholar]
- Miller, R.M. Bioremediation: Science and Applications; Skipper, H.D., Turco, R.F., Eds.; Soil Science Society of America: Medison, WI, USA, 1995; pp. 33–52. [Google Scholar]
- West, C.C.; Harwell, J.H. Surfactants and subsurface remediation. Environ. Sci. Technol 1992, 26, 2324–2330. [Google Scholar]
- Gutierrez, T.; Shimmield, T.; Haidon, C.; Black, K.; Green, D.H. Emulsifying and metal ion binding activity of a glycoprotein exopolymer produced by Pseudoalteromonas sp. strain TG12. Appl. Environ. Microbiol 2008, 74, 4867–4876. [Google Scholar]
- Lester, J.N. Heavy Metals in Wastewater and Sludge Treatment Processes, Vol. II: Treatment and Disposal; CRC Press: Boca Raton, FL, USA, 1987; pp. 15–40. [Google Scholar]
- Prabhukumar, G.; Matsumoto, M.; Mulchandani, A.; Chen, W. Cadmium removal from contaminated soil by tunable biopolymers. Environ. Sci. Technol 2004, 38, 3148–3152. [Google Scholar]
- Torrens, J.L.; Herman, D.C.; Miller-Maier, R.M. Biosurfactant (Rhamnolipid) sorption and the impact on rhamnolipid-facilitated removal of cadmium from various soils under saturated flow conditions. Environ. Sci. Technol 1998, 32, 776–781. [Google Scholar]
- Juwarkar, A.A.; Nair, A.; Dubey, K.V.; Singh, S.K.; Devotta, S. Biosurfactant technology for remediation of cadmium and lead contaminated soils. Chemosphere 2007, 68, 1996–2002. [Google Scholar]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Heavy metal removal from sediments by biosurfactants. J. Hazard. Mater 2001, 85, 111–125. [Google Scholar]
- Chen, W.J.; Hsiao, L.C.; Chen, K.K.Y. Metal desorption from copper(II)/nickel(II)-spiked kaolin as a soil component using plant-derived saponin biosurfactant. Process Biochem 2008, 43, 488–498. [Google Scholar]
- Herman, D.C.; Artiola, J.F.; Miller, R.M. Removal of cadmium, lead, and zinc from soil by a rhamnolipid biosurfactant. Environ. Sci. Technol 1995, 29, 2280–2285. [Google Scholar]
- Zouboulis, A.I.; Matis, K.A.; Lazaridis, N.K.; Golyshin, P.N. The use of biosurfactants in flotation: Application for the removal of metal ions. Miner. Eng 2003, 16, 1231–1236. [Google Scholar]
- Asci, Y.; Nurbas, M.; Acikel, T.S. Sorption of Cd(II) onto kaolin as a soil component and desorption of Cd(II) from kaolin using rhamnolipid biosurfactant. J. Hazard. Mater 2007, 139, 50–56. [Google Scholar]
- Lang, S. Novel Surfactants, Preparation Application and Biodegradability; Holmberg, K., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 279–302. [Google Scholar]
- Martin, P.J.; Dutton, H.M.; Winterburn, J.B.; Baker, S.; Russell, A.B. Foam fractionation with reflux. Chem. Eng. Sci 2010, 65, 3825–3835. [Google Scholar]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F.; James, S.; Bennett, H.P.J. Metal removal from contaminated soil and sediments by the biosurfactant surfactin. Environ. Sci. Technol 1999, 33, 3812–3820. [Google Scholar]
- Heerklotz, H.; Seelig, J. Detergent-like action of the antibiotic peptide surfactin on lipid membranes. Biophys. J 2001, 81, 1547–1554. [Google Scholar]
- Yeom, I.T.; Ghosh, M.M.; Cox, C.D.; Robinson, K.G. Micellar solubilization of polynuclear aromatic hydrocarbons in coal tar-contaminated soils. Environ. Sci. Technol 1995, 29, 3015–3021. [Google Scholar]
- Otto, W.H.; Britten, D.J.; Larive, C.K. NMR diffusion analysis of surfactant-humic substance interactions. J. Colloid Interface Sci 2003, 261, 508–513. [Google Scholar]
- Yeh, M.S.; Wei, Y.H.; Chang, J.S. Bioreactor design for enhanced carrier-assisted surfactin roduction with Bacillus subtilis. Process Biochem 2006, 41, 1799–1805. [Google Scholar]
- Qu, Y.H.; Zeng, G.M.; Huang, J.H.; Xu, K.; Fang, Y.Y.; Li, X.; Liu, H.L. Recovery of surfactant SDS and Cd2+ from permeate in MEUF using a continuous foam fractionator. J. Hazard. Mater 2007, 155, 32–38. [Google Scholar]
- Arima, K.; Kakinuma, A.; Tamura, G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: Isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun 1968, 31, 488–494. [Google Scholar]
- Al-Ajlani, M.; Sheikh, M.A.; Ahmad, Z.; Hasnain, S. Production of surfactin from Bacillus subtilis MZ-7 grown on pharmamedia commercial medium. Microb. Cell Fact 2007, 6, 17:1–17:8. [Google Scholar]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory: New York, NY, USA, 1972. [Google Scholar]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol 2001, 60, 193–207. [Google Scholar]



© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, H.-R.; Chen, C.-C.; Reddy, A.S.; Chen, C.-Y.; Li, W.R.; Tseng, M.-J.; Liu, H.-T.; Pan, W.; Maity, J.P.; Atla, S.B. Removal of Mercury by Foam Fractionation Using Surfactin, a Biosurfactant. Int. J. Mol. Sci. 2011, 12, 8245-8258. https://doi.org/10.3390/ijms12118245
Chen H-R, Chen C-C, Reddy AS, Chen C-Y, Li WR, Tseng M-J, Liu H-T, Pan W, Maity JP, Atla SB. Removal of Mercury by Foam Fractionation Using Surfactin, a Biosurfactant. International Journal of Molecular Sciences. 2011; 12(11):8245-8258. https://doi.org/10.3390/ijms12118245
Chicago/Turabian StyleChen, Hau-Ren, Chien-Cheng Chen, A. Satyanarayana Reddy, Chien-Yen Chen, Wun Rong Li, Min-Jen Tseng, Hung-Tsan Liu, Wei Pan, Jyoti Prakash Maity, and Shashi B. Atla. 2011. "Removal of Mercury by Foam Fractionation Using Surfactin, a Biosurfactant" International Journal of Molecular Sciences 12, no. 11: 8245-8258. https://doi.org/10.3390/ijms12118245



