Exploring the Immunoproteome for Ovarian Cancer Biomarker Discovery
Abstract
:1. Introduction
2. Biomarkers
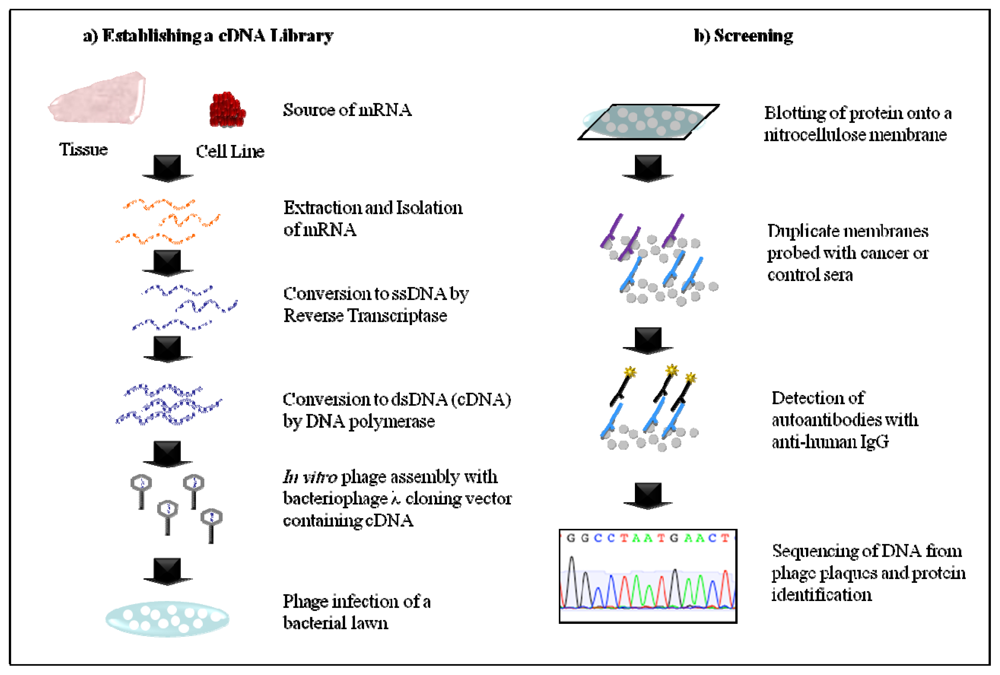
2.1. Serological Analysis of Recombinant cDNA Expression Libraries (SEREX)
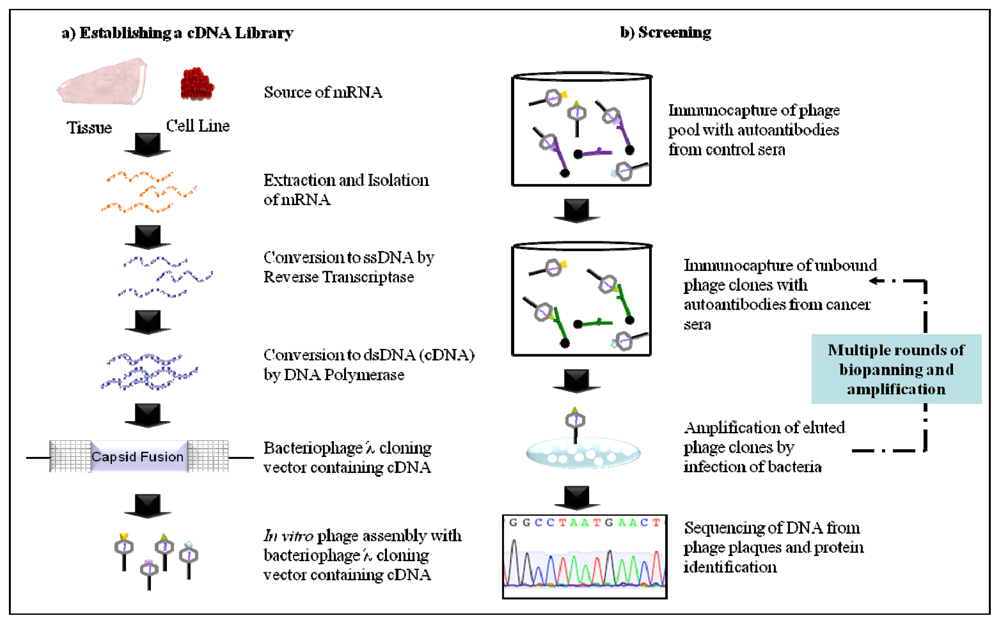
2.2. Phage Display
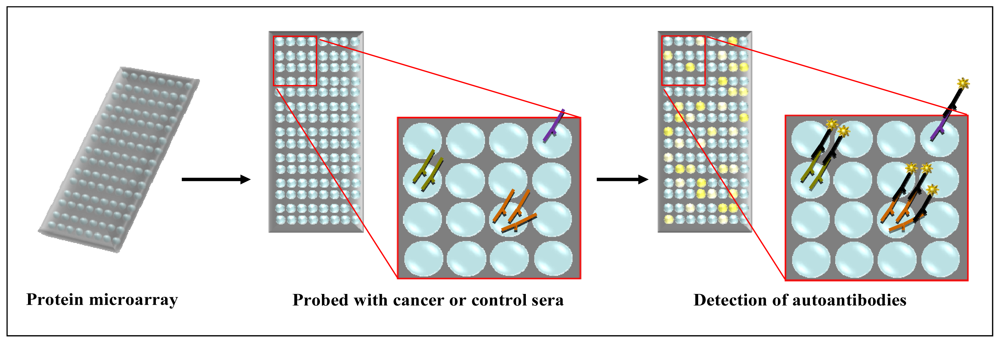
2.3. Protein Microarray
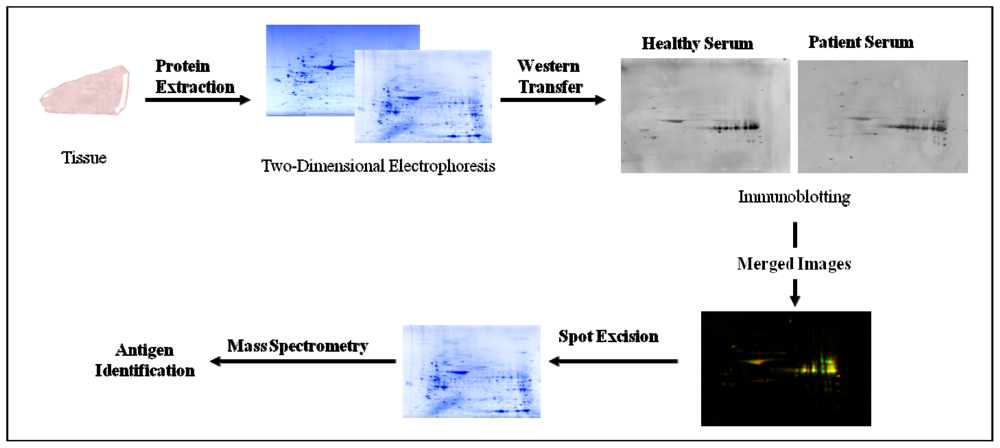
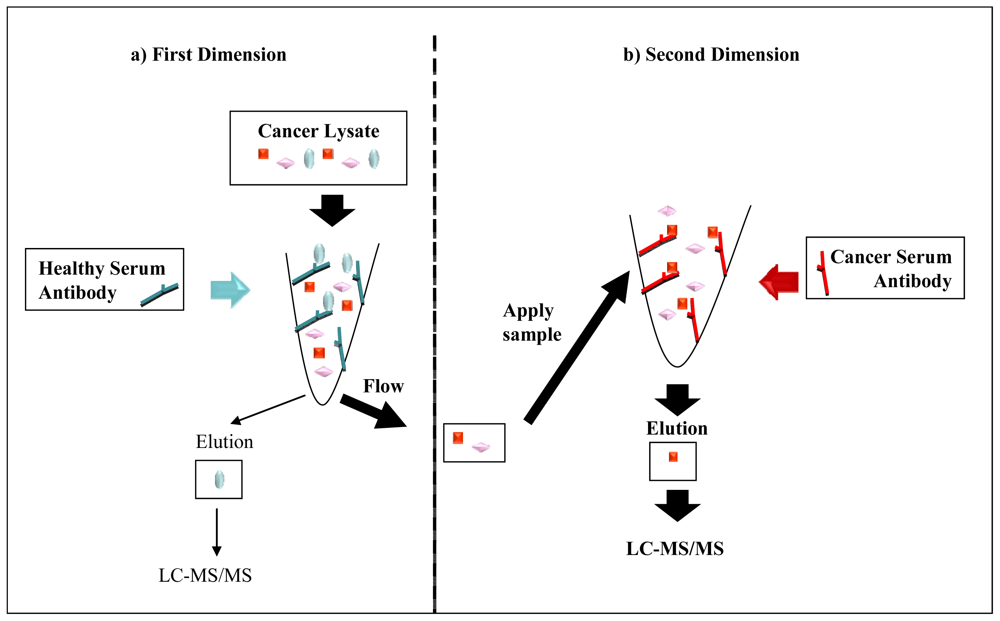
2.4. Serological Proteome Analysis (SEPRA)
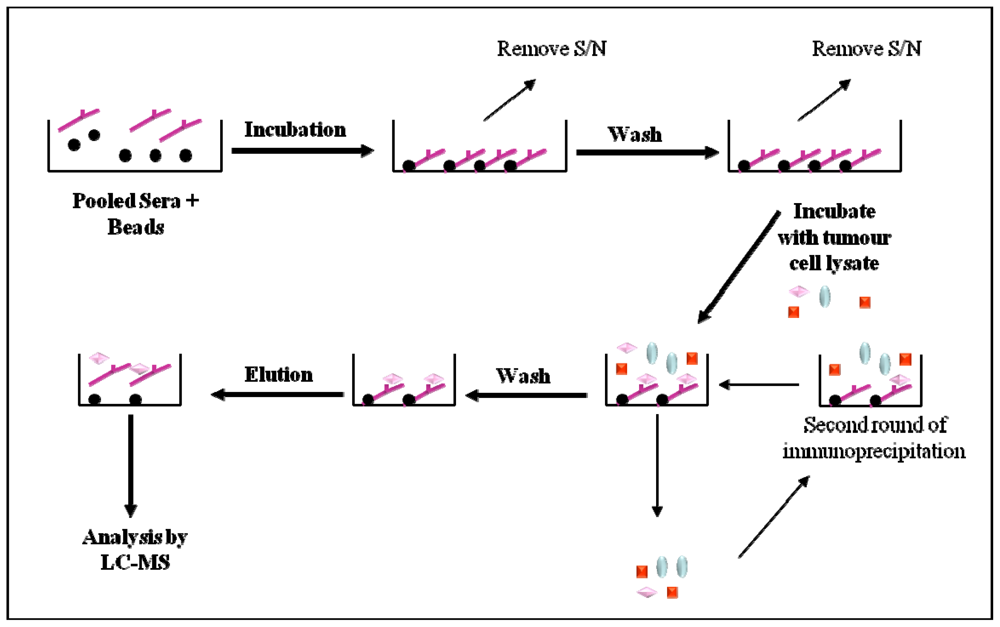
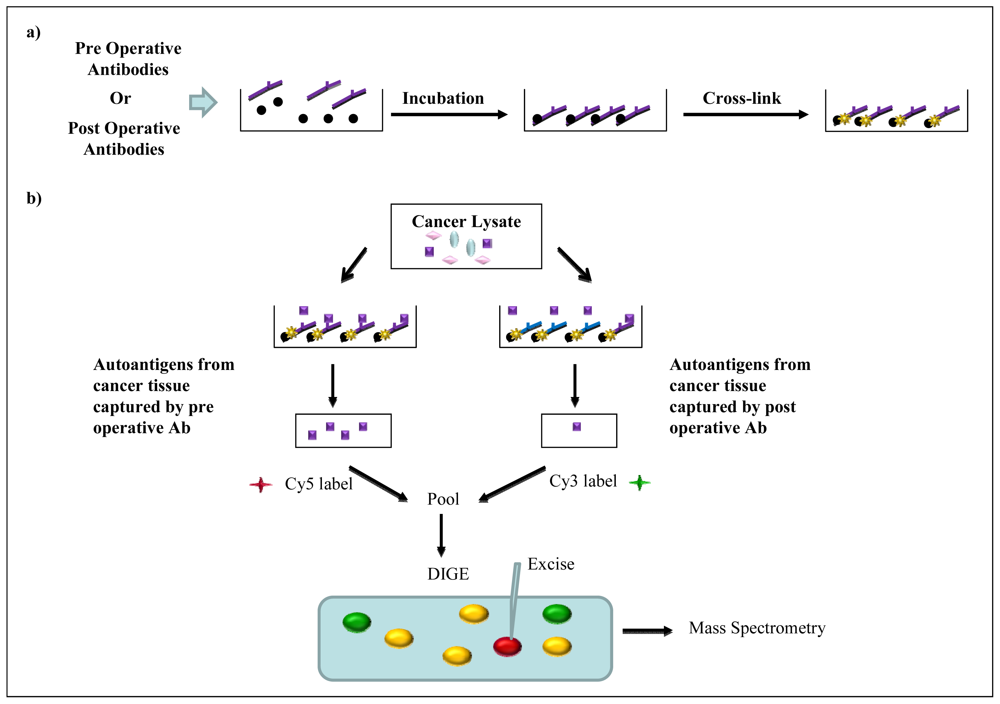
2.5. Immunoaffinity Purification Methods
3. Conclusion
Acknowledgement
Abbreviations
| 2-DE | two-dimensional electrophoresis |
| 2D-DITA | two-dimensional differential gel electrophoresis analysis of immuno-precipitated tumour antigens |
| DIGE | differential gel electrophoresis |
| ELISA | enzyme-linked immunosorbent assay |
| EST | expressed sequence tag |
| FIGO | federation of gynecologists & obstetricians |
| LC | liquid chromatography |
| MS | mass spectrometry |
| SEPRA | serological proteome analysis |
| SEREX | Serological analysis of recombinant cDNA expression libraries |
| TVU | transvaginal ultrasonography |
| TAA | tumour associated antigen |
| PPV | positive predictive value |
| PTM | post translational modification |
References
- Ovarian Cancer in Australia: An Overview, 2010. In Cancer series no. 52; Australian Institute of Health and Welfare and National Breast and Ovarian Cancer Centre: Canberra, Australia, February 2010.
- Altekruse, SF; Kosary, CL; Krapcho, M; Neyman, N; Aminou, R; Waldron, W; Ruhl, J; Howlader, N; Tatalovich, Z; Cho, H; Mariotto, A; Eisner, MP; Lewis, DR; Cronin, K; Chen, HS; Feuer, EJ; Stinchcomb, DG; Edwards, BK (Eds.) SEER Cancer Statistics Review 1975–2007; National Cancer Institute: Bethesda, MD, USA, 2011.
- Hennessy, BT; Coleman, RL; Markman, M. Ovarian cancer. Lancet 2009, 374, 1371–1382. [Google Scholar]
- Guarneri, V; Piacentini, F; Barbieri, E; Conte, PF. Achievements and unmet needs in the management of advanced ovarian cancer. Gynecol. Oncol 2010, 117, 152–158. [Google Scholar]
- Menon, U; Gentry-Maharaj, A; Hallett, R; Ryan, A; Burnell, M; Sharma, A; Lewis, S; Davies, S; Philpott, S; Lopes, A; et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol 2009, 10, 327–340. [Google Scholar]
- Badgwell, D; Bast, RC, Jr. Early detection of ovarian cancer. Dis Markers 2007, 23, 397–410. [Google Scholar]
- MacDonald, ND; Rosenthal, AN; Jacobs, IJ. Screening for ovarian cancer. Ann. Acad. Med Singapore 1998, 27, 676–682. [Google Scholar]
- Bast, RC, Jr; Feeney, M; Lazarus, H; Nadler, LM; Colvin, RB; Knapp, RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Invest 1981, 68, 1331–1337. [Google Scholar]
- Bast, RC, Jr; Klug, TL; St John, E; Jenison, E; Niloff, JM; Lazarus, H; Berkowitz, RS; Leavitt, T; Griffiths, CT; Parker, L; Zurawski, VR, Jr; Knapp, RC. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N. Engl. J. Med 1983, 309, 883–887. [Google Scholar]
- Mann, WJ; Patsner, B; Cohen, H; Loesch, M. Preoperative serum CA-125 levels in patients with surgical stage I invasive ovarian adenocarcinoma. J. Natl. Cancer Inst 1988, 80, 208–209. [Google Scholar]
- Malkasian, GD, Jr; Knapp, RC; Lavin, PT; Zurawski, VR, Jr; Podratz, KC; Stanhope, CR; Mortel, R; Berek, JS; Bast, RC, Jr; Ritts, RE. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am. J. Obstet. Gynecol 1988, 159, 341–346. [Google Scholar]
- Bast, RC, Jr; Badgwell, D; Lu, Z; Marquez, R; Rosen, D; Liu, J; Baggerly, KA; Atkinson, EN; Skates, S; Zhang, Z; Lokshin, A; Menon, U; Jacobs, I; Lu, K. New tumor markers: CA125 and beyond. Int. J. Gynecol Cancer 2005, 15, 274–281. [Google Scholar]
- Anderson, NL; Anderson, NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics 2002, 1, 845–867. [Google Scholar]
- Tan, HT; Low, J; Lim, SG; Chung, MC. Serum autoantibodies as biomarkers for early cancer detection. FEBS J 2009, 276, 6880–6904. [Google Scholar]
- Traggiai, E; Puzone, R; Lanzavecchia, A. Antigen dependent and independent mechanisms that sustain serum antibody levels. Vaccine 2003, 21, S35–S37. [Google Scholar]
- Slifka, MK; Ahmed, R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr. Opin. Immunol 1998, 10, 252–258. [Google Scholar]
- Morell, A; Terry, WD; Waldmann, TA. Metabolic properties of IgG subclasses in man. J. Clin. Invest 1970, 49, 673–680. [Google Scholar]
- Chen, YT. Cancer vaccine: identification of human tumor antigens by SEREX. Cancer J 2000, 6, S208–S217. [Google Scholar]
- Stone, B; Schummer, M; Paley, PJ; Thompson, L; Stewart, J; Ford, M; Crawford, M; Urban, N; O'Briant, K; Nelson, BH. Serologic analysis of ovarian tumor antigens reveals a bias toward antigens encoded on 17q. Int. J Cancer 2003, 104, 73–84. [Google Scholar]
- Luo, LY; Herrera, I; Soosaipillai, A; Diamandis, EP. Identification of heat shock protein 90 and other proteins as tumour antigens by serological screening of an ovarian carcinoma expression library. Br. J Cancer 2002, 87, 339–343. [Google Scholar]
- Lokshin, AE; Winans, M; Landsittel, D; Marrangoni, AM; Velikokhatnaya, L; Modugno, F; Nolen, BM; Gorelik, E. Circulating IL-8 and anti-IL-8 autoantibody in patients with ovarian cancer. Gynecol. Oncol 2006, 102, 244–251. [Google Scholar]
- Jin, S; Wang, Y; Zhang, Y; Zhang, HZ; Wang, SJ; Tang, JQ; Chen, HJ; Ge, HL. Humoral immune responses against tumor-associated antigen OVA66 originally defined by serological analysis of recombinant cDNA expression libraries and its potentiality in cellular immunity. Cancer Sci 2008, 99, 1670–1678. [Google Scholar]
- The Academy of Cancer Immunology and the Ludwig Institute for Cancer Research. Cancer Immunome Database. 1997. Avaible online: http://ludwig-sun5.unil.ch/CancerImmunomeDB (accessed on January 13, 2011).
- Vidal, CI; Mintz, PJ; Lu, K; Ellis, LM; Manenti, L; Giavazzi, R; Gershenson, DM; Broaddus, R; Liu, J; Arap, W; Pasqualini, R. An HSP90-mimic peptide revealed by fingerprinting the pool of antibodies from ovarian cancer patients. Oncogene 2004, 23, 8859–8867. [Google Scholar]
- Chatterjee, M; Mohapatra, S; Ionan, A; Bawa, G; Ali-Fehmi, R; Wang, X; Nowak, J; Ye, B; Nahhas, FA; Lu, K; Witkin, SS; Fishman, D; Munkarah, A; Morris, R; Levin, NK; Shirley, NN; Tromp, G; Abrams, J; Draghici, S; Tainsky, MA. Diagnostic markers of ovarian cancer by high-throughput antigen cloning and detection on arrays. Cancer Res 2006, 66, 1181–1190. [Google Scholar]
- Hudson, ME; Pozdnyakova, I; Haines, K; Mor, G; Snyder, M. Identification of differentially expressed proteins in ovarian cancer using high-density protein microarrays. Proc. Natl. Acad. Sci USA 2007, 104, 17494–17499. [Google Scholar]
- Gunawardana, CG; Memari, N; Diamandis, EP. Identifying novel autoantibody signatures in ovarian cancer using high-density protein microarrays. Clin. Biochem 2009, 42, 426–429. [Google Scholar]
- Taylor, DD; Gercel-Taylor, C; Parker, LP. Patient-derived tumor-reactive antibodies as diagnostic markers for ovarian cancer. Gynecol. Oncol 2009, 115, 112–120. [Google Scholar]
- Philip, R; Murthy, S; Krakover, J; Sinnathamby, G; Zerfass, J; Keller, L; Philip, M. Shared immunoproteome for ovarian cancer diagnostics and immunotherapy: potential theranostic approach to cancer. J. Proteome Res 2007, 6, 2509–2517. [Google Scholar]
- Gagnon, A; Kim, JH; Schorge, JO; Ye, B; Liu, B; Hasselblatt, K; Welch, WR; Bandera, CA; Mok, SC. Use of a combination of approaches to identify and validate relevant tumor-associated antigens and their corresponding autoantibodies in ovarian cancer patients. Clin. Cancer Res 2008, 14, 764–771. [Google Scholar]
- Kim, S; Cho, H; Nam, EJ; Kim, SW; Kim, YT; Park, YW; Kim, BW; Kim, JH. Autoantibodies against stress-induced phosphoprotein-1 as a novel biomarker candidate for ovarian cancer. Genes Chromosomes Cancer 2010, 49, 585–595. [Google Scholar]
- Kijanka, G; Murphy, D. Protein arrays as tools for serum autoantibody marker discovery in cancer. J Proteomics 2009, 72, 936–944. [Google Scholar]
- Kricka, LJ; Master, SR; Joos, TO; Fortina, P. Current perspectives in protein array technology. Ann. Clin. Biochem 2006, 43, 457–467. [Google Scholar]
- Gnjatic, S; Ritter, E; Buchler, MW; Giese, NA; Brors, B; Frei, C; Murray, A; Halama, N; Zornig, I; Chen, YT; Andrews, C; Ritter, G; Old, LJ; Odunsi, K; Jager, D. Seromic profiling of ovarian and pancreatic cancer. Proc. Natl. Acad. Sci USA 2010, 107, 5088–5093. [Google Scholar]
- Ramachandran, N; Hainsworth, E; Bhullar, B; Eisenstein, S; Rosen, B; Lau, AY; Walter, JC; LaBaer, J. Self-assembling protein microarrays. Science 2004, 305, 86–90. [Google Scholar]
- Madoz-Gurpide, J; Wang, H; Misek, DE; Brichory, F; Hanash, SM. Protein based microarrays: a tool for probing the proteome of cancer cells and tissues. Proteomics 2001, 1, 1279–1287. [Google Scholar]
- Nam, MJ; Madoz-Gurpide, J; Wang, H; Lescure, P; Schmalbach, CE; Zhao, R; Misek, DE; Kuick, R; Brenner, DE; Hanash, SM. Molecular profiling of the immune response in colon cancer using protein microarrays: occurrence of autoantibodies to ubiquitin C-terminal hydrolase L3. Proteomics 2003, 3, 2108–2115. [Google Scholar]
- Qiu, J; Madoz-Gurpide, J; Misek, DE; Kuick, R; Brenner, DE; Michailidis, G; Haab, BB; Omenn, GS; Hanash, S. Development of natural protein microarrays for diagnosing cancer based on an antibody response to tumor antigens. J. Proteome Res 2004, 3, 261–267. [Google Scholar]
- Kopf, E; Zharhary, D. Antibody arrays—an emerging tool in cancer proteomics. Int. J. Biochem. Cell Biol 2007, 39, 1305–1317. [Google Scholar]
- Qin, S; Qiu, W; Ehrlich, JR; Ferdinand, AS; Richie, JP; O'Leary, MP; Lee, ML; Liu, BC. Development of a “reverse capture” autoantibody microarray for studies of antigen-autoantibody profiling. Proteomics 2006, 6, 3199–3209. [Google Scholar]
- Zolg, W. The proteomic search for diagnostic biomarkers: lost in translation? Mol. Cell Proteomics 2006, 5, 1720–1726. [Google Scholar]
- Diz, AP; Truebano, M; Skibinski, DO. The consequences of sample pooling in proteomics: an empirical study. Electrophoresis 2009, 30, 2967–2975. [Google Scholar]
- Friedman, DB; Hoving, S; Westermeier, R. Isoelectric focusing and two-dimensional gel electrophoresis. Methods Enzymol 2009, 463, 515–540. [Google Scholar]
- Gorg, A; Weiss, W; Dunn, MJ. Current two-dimensional electrophoresis technology for proteomics. Proteomics 2004, 4, 3665–3685. [Google Scholar]
- Klade, CS; Voss, T; Krystek, E; Ahorn, H; Zatloukal, K; Pummer, K; Adolf, GR. Identification of tumor antigens in renal cell carcinoma by serological proteome analysis. Proteomics 2001, 1, 890–898. [Google Scholar]
- Yang, F; Xiao, ZQ; Zhang, XZ; Li, C; Zhang, PF; Li, MY; Chen, Y; Zhu, GQ; Sun, Y; Liu, YF; Chen, ZC. Identification of tumor antigens in human lung squamous carcinoma by serological proteome analysis. J. Proteome Res 2007, 6, 751–758. [Google Scholar]
- Tsunemi, S; Nakanishi, T; Fujita, Y; Bouras, G; Miyamoto, Y; Miyamoto, A; Nomura, E; Takubo, T; Tanigawa, N. Proteomics-based identification of a tumor-associated antigen and its corresponding autoantibody in gastric cancer. Oncol. Rep 2010, 23, 949–956. [Google Scholar]
- Hamrita, B; Chahed, K; Kabbage, M; Guillier, CL; Trimeche, M; Chaieb, A; Chouchane, L. Identification of tumor antigens that elicit a humoral immune response in breast cancer patients' sera by serological proteome analysis (SERPA). Clin. Chim Acta 2008, 393, 95–102. [Google Scholar]
- Desmetz, C; Bibeau, F; Boissiere, F; Bellet, V; Rouanet, P; Maudelonde, T; Mange, A; Solassol, J. Proteomics-based identification of HSP60 as a tumor-associated antigen in early stage breast cancer and ductal carcinoma in situ. J. Proteome Res 2008, 7, 3830–3837. [Google Scholar]
- Xia, Q; Kong, XT; Zhang, GA; Hou, XJ; Qiang, H; Zhong, RQ. Proteomics-based identification of DEAD-box protein 48 as a novel autoantigen, a prospective serum marker for pancreatic cancer. Biochem. Biophys. Res. Commun 2005, 330, 526–532. [Google Scholar]
- Imafuku, Y; Omenn, GS; Hanash, S. Proteomics approaches to identify tumor antigen directed autoantibodies as cancer biomarkers. Dis Markers 2004, 20, 149–153. [Google Scholar]
- Barua, A; Bradaric, MJ; Kebede, T; Espionosa, S; Edassery, SL; Bitterman, P; Rotmensch, J; Luborsky, JL. Anti-tumor and anti-ovarian autoantibodies in women with ovarian cancer. Am. J. Reprod. Immunol 2007, 57, 243–249. [Google Scholar]
- Luborsky, JL; Barua, A; Shatavi, SV; Kebede, T; Abramowicz, J; Rotmensch, J. Anti-tumor antibodies in ovarian cancer. Am. J. Reprod. Immunol 2005, 54, 55–62. [Google Scholar]
- Caron, M; Choquet-Kastylevsky, G; Joubert-Caron, R. Cancer immunomics using autoantibody signatures for biomarker discovery. Mol Cell Proteomics 2007, 6, 1115–1122. [Google Scholar]
- Hardouin, J; Lasserre, JP; Sylvius, L; Joubert-Caron, R; Caron, M. Cancer immunomics: from serological proteome analysis to multiple affinity protein profiling. Ann. N. Y. Acad. Sci 2007, 1107, 223–230. [Google Scholar]
- Hardouin, J; Lasserre, JP; Canelle, L; Duchateau, M; Vlieghe, C; Choquet-Kastylevsky, G; Joubert-Caron, R; Caron, M. Usefulness of autoantigens depletion to detect autoantibody signatures by multiple affinity protein profiling. J. Sep. Sci 2007, 30, 352–358. [Google Scholar]
| Technique | Antigen Source (cDNA Library) | Autoantibody Source | TAA | Ref. |
|---|---|---|---|---|
| SEREX |
| Serum | p53 NY-ESO-1 Topoisomerase IIα Ubiquilin-1 Homeobox B6 HMBA inducible protein HDCMA 18P protein | [19] |
| Commercial cDNA library | Ascites | HSP90 | [20] | |
| Commercial cDNA library | Serum | IL-8 | [21] | |
| Ovarian cancer tumour lysate | Serum | OVA66 | [22] | |
| Phage Display | Random peptide library | Ascites | Mimic peptide CVPELGHEC | [24] |
| Ovarian cancer cell line | Serum | RCAS1 Signal recognition protein-19 AHNAK-related sequence NASP Nijmegen breakage syndrome 1 Ribosomal protein L4 Homo Sapiens KIAA0419 gene product Eukaryotic initiation factor 5A Casein kinase II Chromodomain helicase DNA-binding protein 1 | [25] | |
| Protein Microarray | ProtoArray (V3.0) | Serum | 94 Autoantigens | [26] |
| ProtoArray (V4.0) | Ascites | L-aminoadipate-semialdehyde dehydrogenase-phosphopantetheinyl transferase (AASDHPPT) | [27] | |
| Ovarian cancer cell line (exosome derived) dot blot array | Serum | Nucleophosmin Cathepsin D SSX common antigen GRP78 P53 Placental-type alkaline phosphatase Heat shock protein 90 NY-ESO-1 Survivin TAG72 CA125 HoxA7 | [28] | |
| Immunoaffinity Purification Methods | Ovarian adenocarcinoma cell line lysate | Serum | A-kinase anchor protein 9 Eukaryotic translation initiation factor 4γ Midasian RAD50 Talin1 Vinculin Vimention Centrosome-associated protein 350 | [29] |
| Malignant ovarian tissue lysate | Plasma | S100A7 | [30] | |
| Ovarian tissue lysate | Serum | Stress-induced phosphoprotein-1 | [31] |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martin, K.; Ricciardelli, C.; Hoffmann, P.; Oehler, M.K. Exploring the Immunoproteome for Ovarian Cancer Biomarker Discovery. Int. J. Mol. Sci. 2011, 12, 410-428. https://doi.org/10.3390/ijms12010410
Martin K, Ricciardelli C, Hoffmann P, Oehler MK. Exploring the Immunoproteome for Ovarian Cancer Biomarker Discovery. International Journal of Molecular Sciences. 2011; 12(1):410-428. https://doi.org/10.3390/ijms12010410
Chicago/Turabian StyleMartin, Karina, Carmela Ricciardelli, Peter Hoffmann, and Martin K. Oehler. 2011. "Exploring the Immunoproteome for Ovarian Cancer Biomarker Discovery" International Journal of Molecular Sciences 12, no. 1: 410-428. https://doi.org/10.3390/ijms12010410
APA StyleMartin, K., Ricciardelli, C., Hoffmann, P., & Oehler, M. K. (2011). Exploring the Immunoproteome for Ovarian Cancer Biomarker Discovery. International Journal of Molecular Sciences, 12(1), 410-428. https://doi.org/10.3390/ijms12010410





