Acetylene-Based Materials in Organic Photovoltaics
Abstract
:1. Introduction
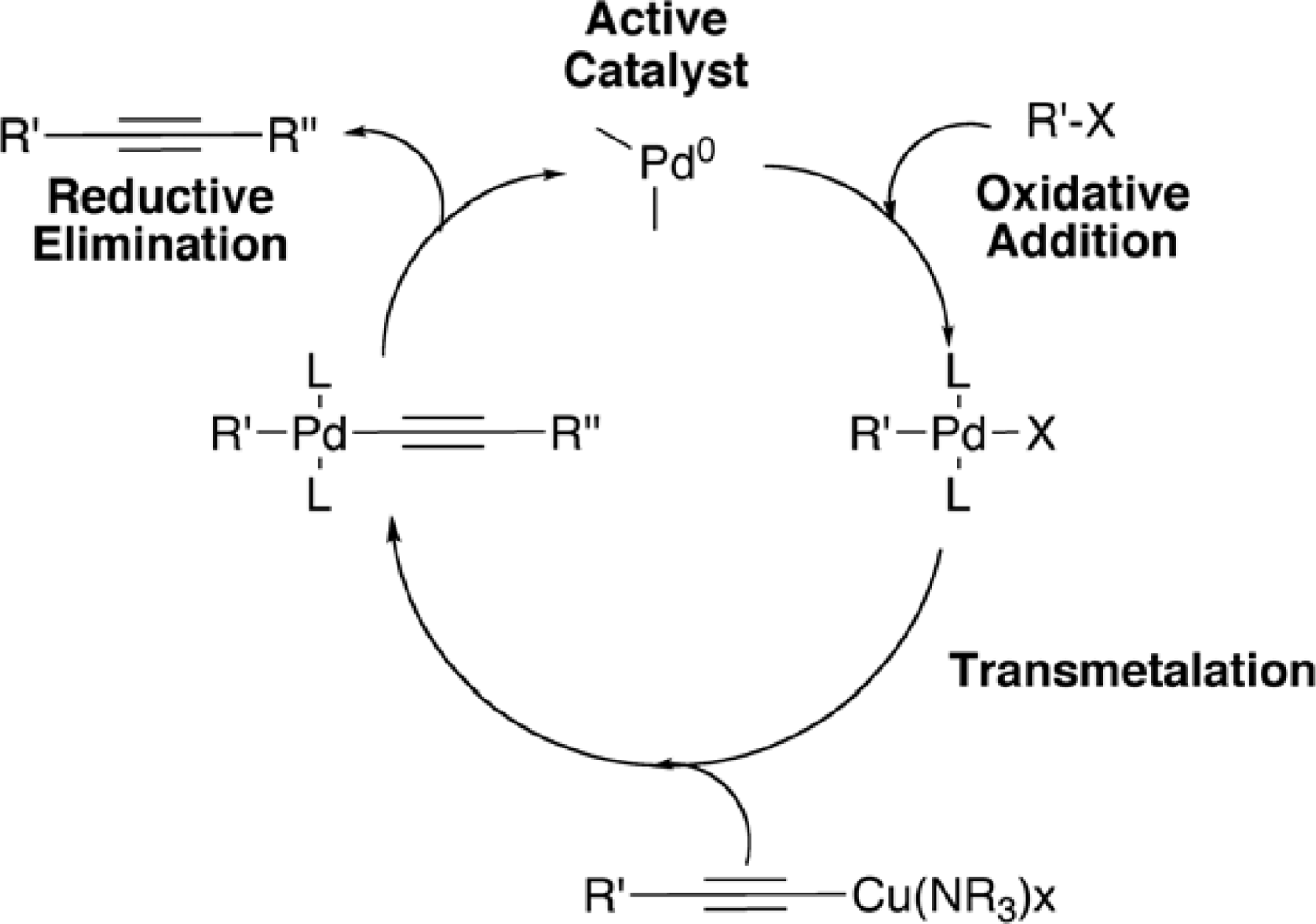
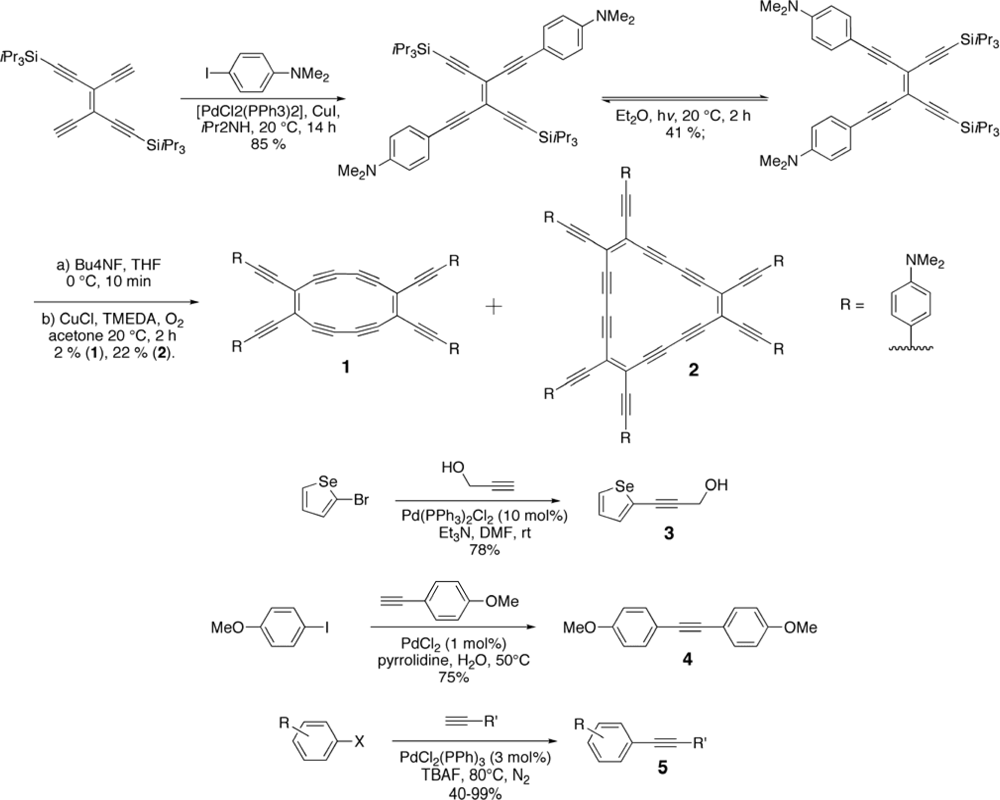
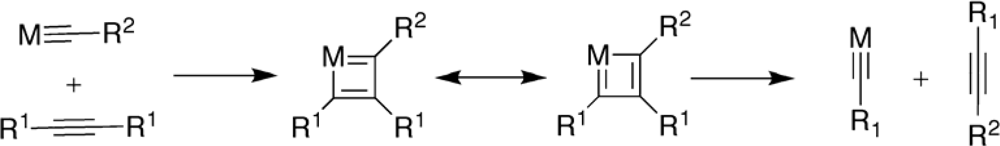
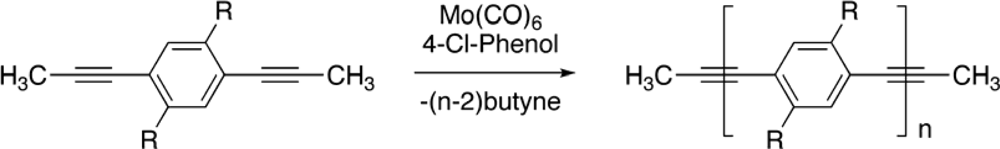
2. Synthesis
3. Photovoltaic Studies
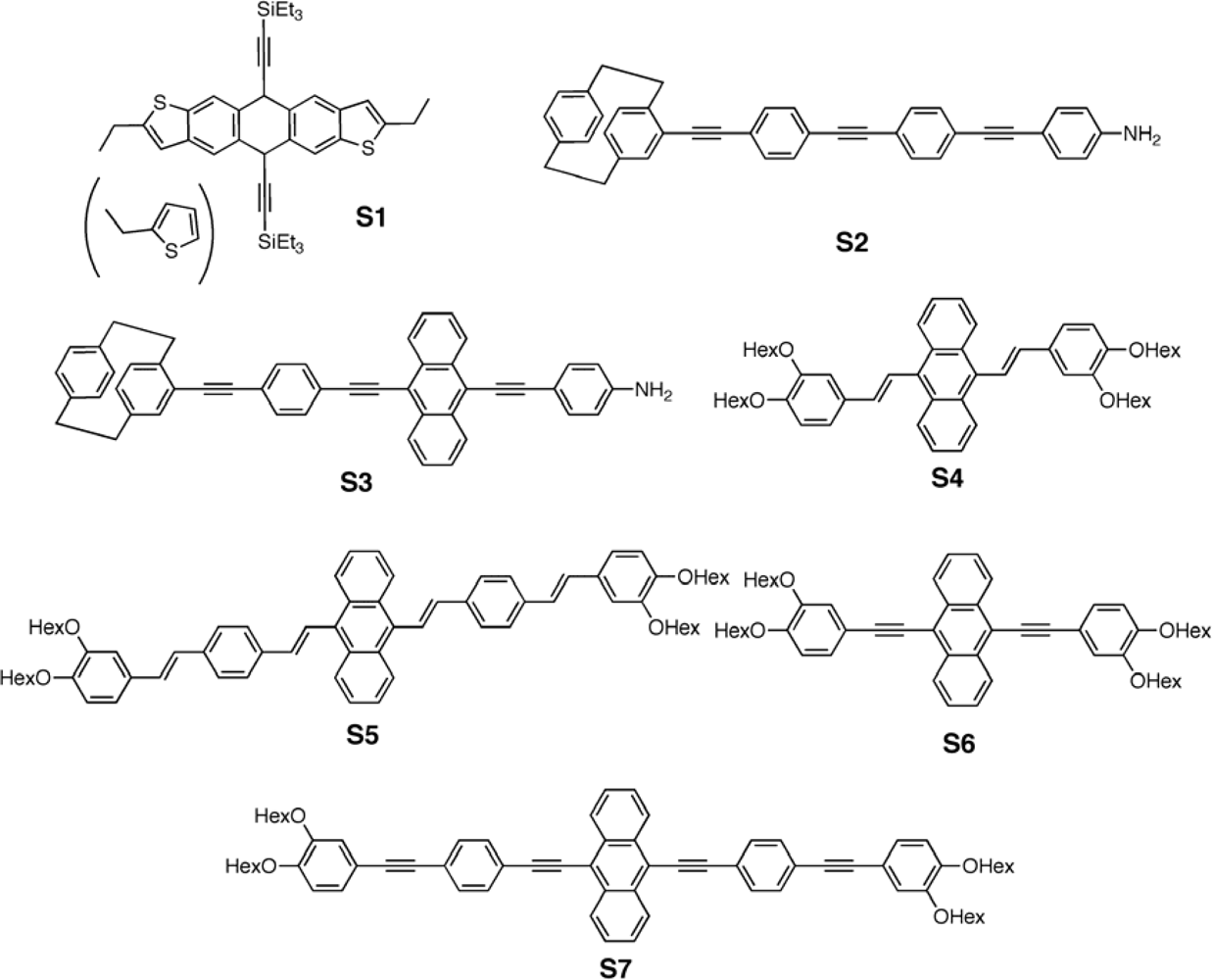
3.1. Small Molecules
3.2. (Co)Polymers and Metallopolyyne Polymers
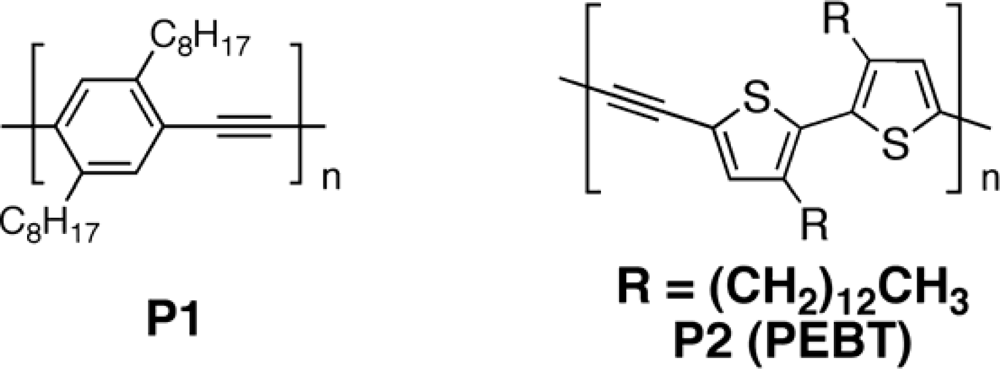
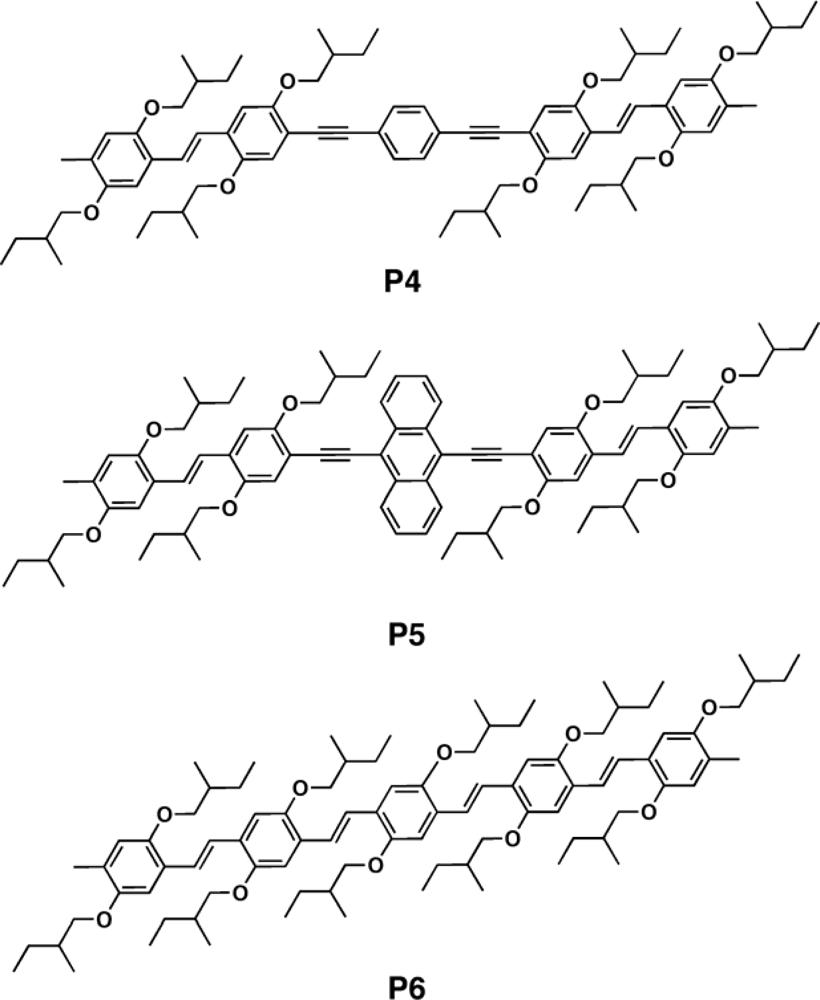
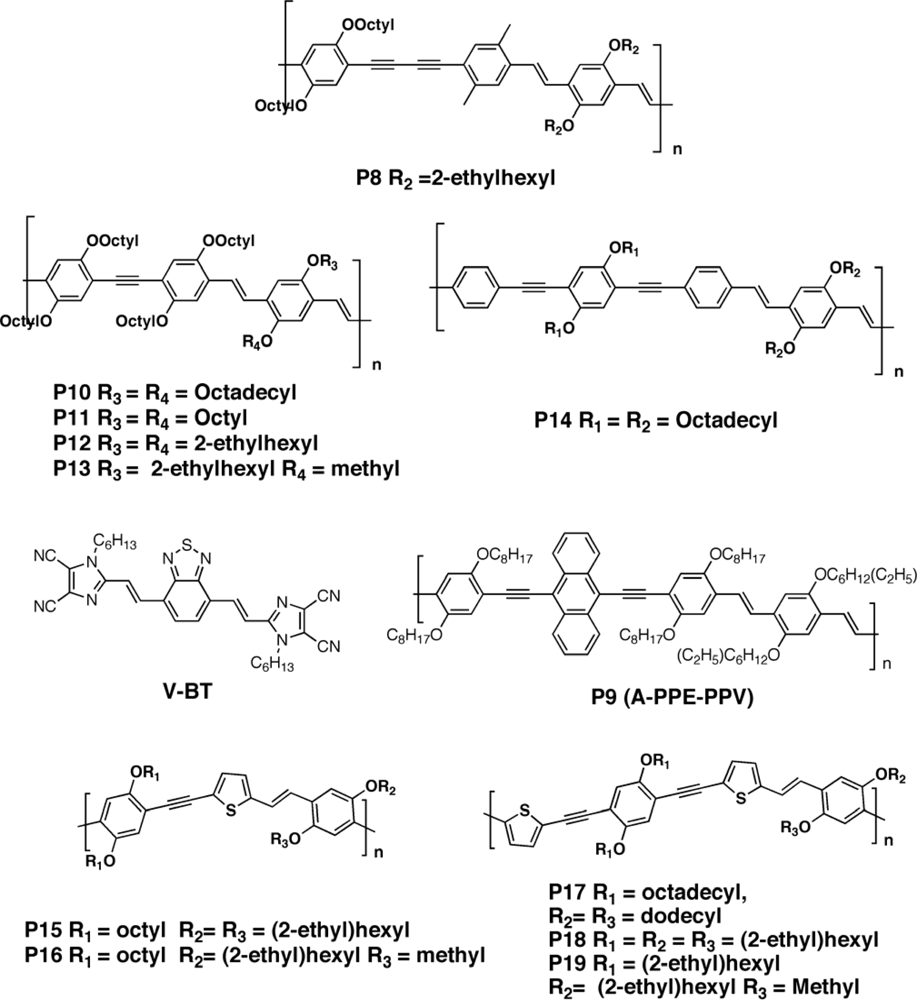
3.2.1. Polymers
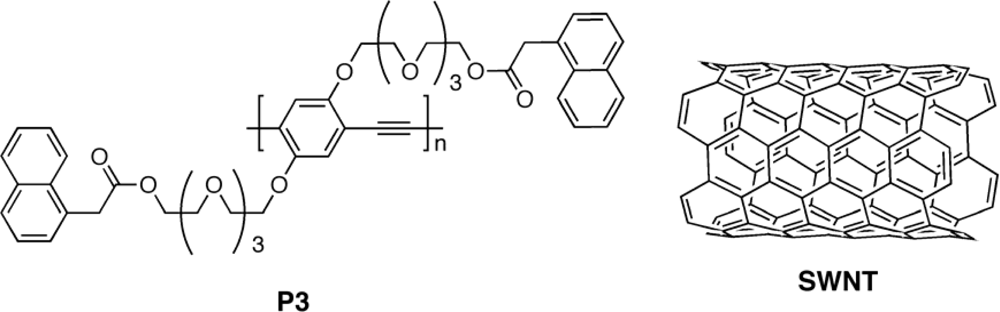
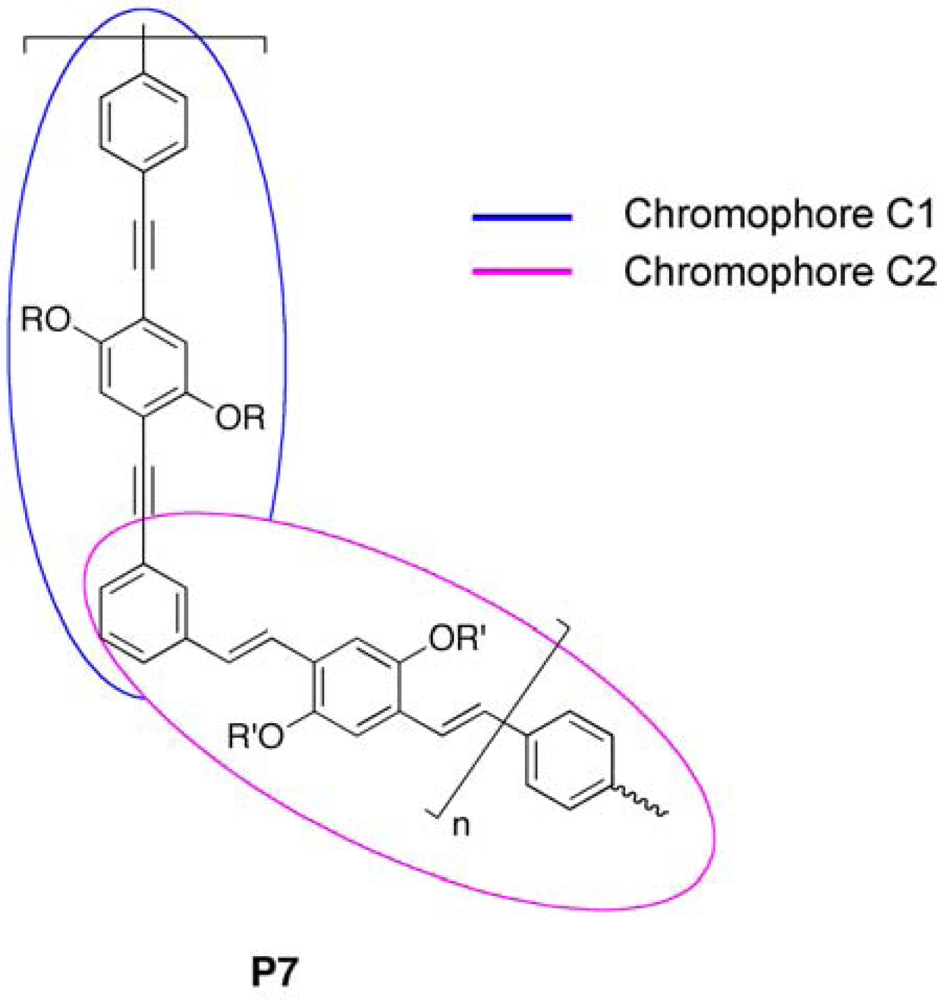
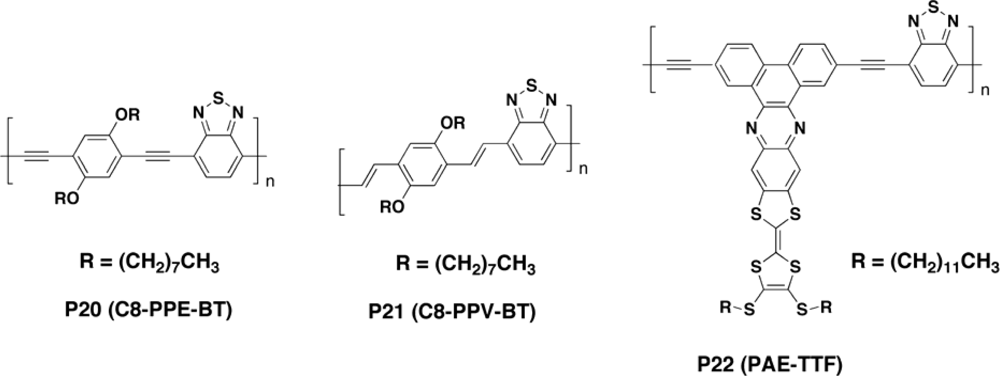
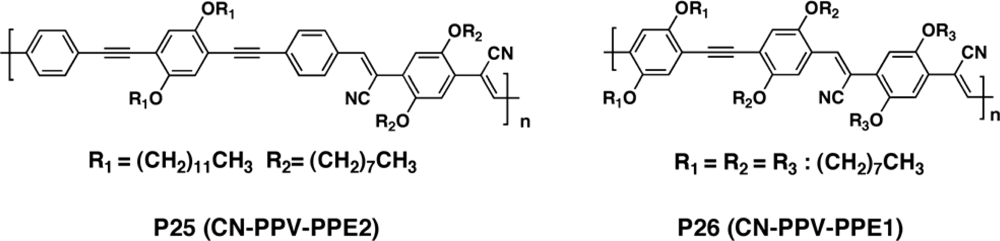
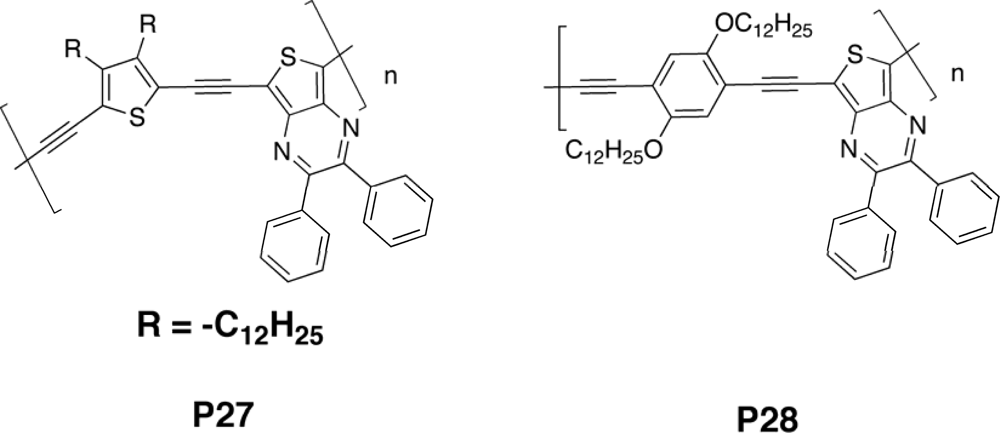
3.2.2. Copolymers
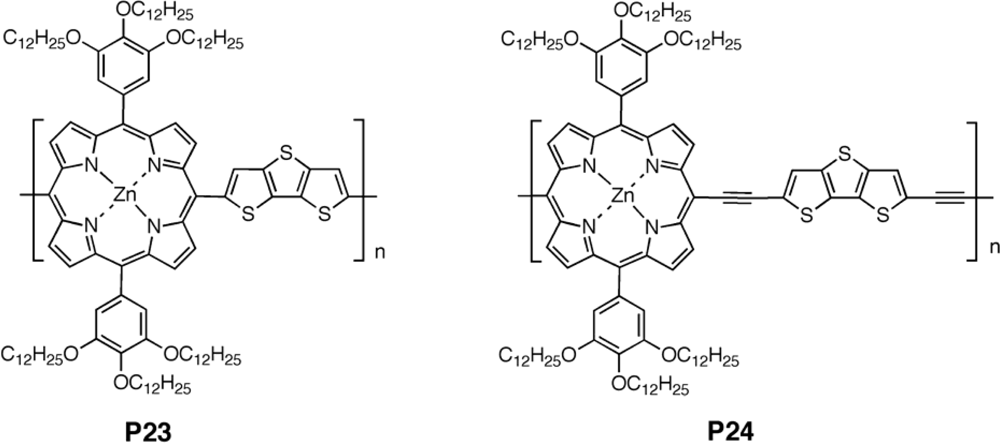
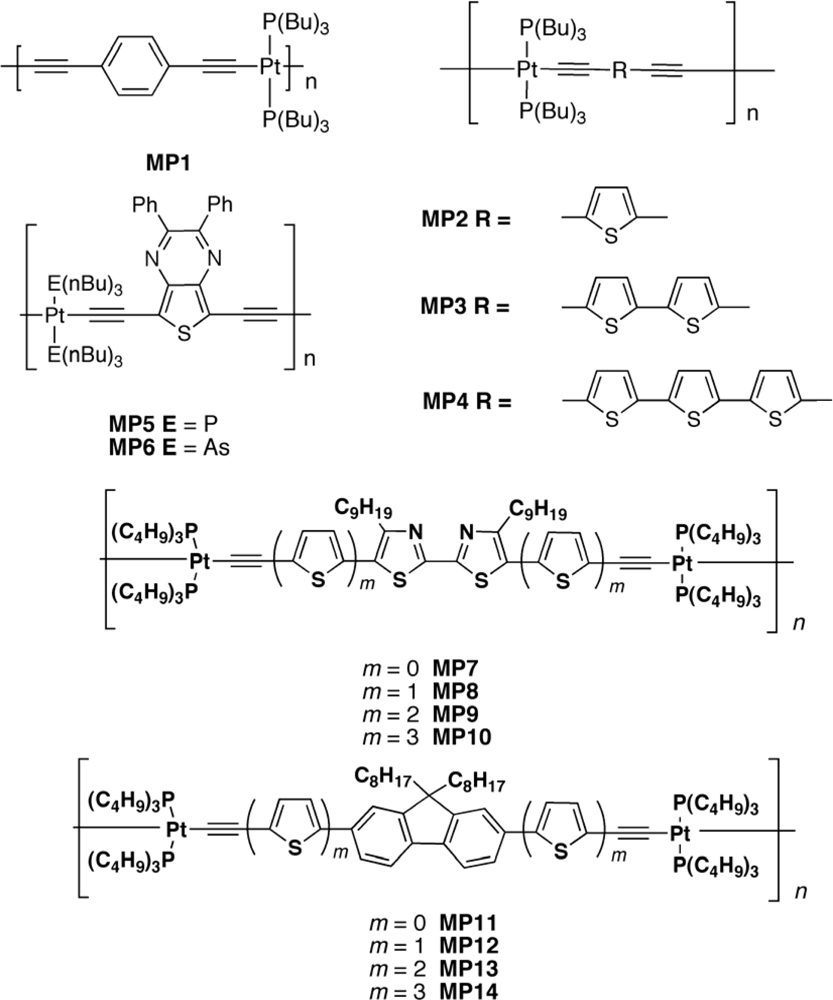
3.2.3. Metallopolyyne Polymers
3.3. Donor-Fullerene Hybrids
4. Conclusions
References and Notes
- Zhao, L; Zou, JH; Huang, J; Li, C; Zhang, Y; Sun, C; Zhu, XH; Peng, J; Cao, Y; Roncali, J. Asymmetrically 9,10-disubstituted anthracenes as soluble and stable blue electroluminescent molecular glasses. Org. El 2008, 9, 649–655. [Google Scholar] [Green Version]
- Luo, J; Zhou, Y; Niu, ZQ; Zhou, QF; Ma, Y; Pei, J. Three-dimensional architectures for highly stable pure blue emission. J. Am. Chem. Soc 2007, 129, 11314–11315. [Google Scholar]
- Tang, ML; Mannsfeld, SCB; Sun, Y-S; Becerril, HA; Bao, Z. Pentaceno[2,3-b]thiophene, a hexacene analog for organic thin film transistors. J. Am. Chem. Soc 2009, 131, 882–883. [Google Scholar]
- Xia, Y; Cho, J; Paulsen, B; Frisbie, CD; Renn, MJ. Correlation of on-state conductance with referenced electrochemical potential in ion gel gated polymer transistors. Appl Phys Lett 2009, 94, 013304/1–013304/3. [Google Scholar]
- Allard, S; Forster, M; Souharce, B; Thiem, H; Sherf, U. Organic Semiconductors for Solution-Processable Field-Effect Transistors (OFETs). Angew. Chem. Int. Ed 2008, 47, 4070–4098. [Google Scholar]
- Liang, Y; Feng, D; Guo, J; Szarko, JM; Ray, C; Chen, LX; Yu, L. Regioregular Oligomer and Polymer Containing Thieno[3,4-b]thiophene Moiety for Efficient Organic Solar Cells. Macromolecules 2009, 42, 1091–1098. [Google Scholar]
- Ooi, ZE; Tam, TL; Shin, RYC; Chen, ZK; Kietzke, T; Sellinger, A; Baumgarten, M; Müllen, K; deMello, J. Solution processable bulk-heterojunction solar cells using a small molecule acceptor. J. Mater. Chem 2008, 18, 4619–4622. [Google Scholar]
- Wei, SKH; Chen, SH; Dolgaleva, K; Lukishova, S; Boyd, RW. Robust organic lasers comprising glassy-cholesteric pentafluorene doped with a red-emitting oligofluorene. Appl Phys Lett 2009, 94, 041111/1–041111/3. [Google Scholar]
- Amarasinghe, D; Ruseckas, A; Vasdekis, AE; Turnbull, GA; Samuel, IDW. Picosecond gain switching of an organic semiconductor optical amplifier. Appl Phys Lett 2008, 92, 083305/1–083305/3. [Google Scholar]
- Silvestri, F; Irwin, MD; Beverina, L; Facchetti, A; Pagani, GA; Marks, TJ. Efficient Squaraine-Based Solution Processable Bulk-Heterojunction Solar Cells. J. Am. Chem. Soc 2008, 130, 17640–17641. [Google Scholar]
- Bagnis, D; Beverina, L; Huang, H; Silvestri, F; Yao, Y; Yan, H; Pagani, GA; Marks, TJ; Facchetti, A. Marked Alkyl- vs Alkenyl-Substituent Effects on Squaraine Dye Solid-State Structure, Carrier Mobility, and Bulk Heterojunction Solar Cells Efficiency. J. Am. Chem. Soc 2010, 132, 4074–4075. [Google Scholar]
- Moliton, A; Nunzi, J-M. How to model the behaviour of organic photovoltaics cells. Polym. Int 2006, 55, 583–600. [Google Scholar]
- Chen, CP; Chan, SH; Chao, TC; Ting, C; Ko, BT. Low-Bandgap Poly(Thiophene-Phenylene-Thiophene) Derivatives with Broaden Absorption Spectra for Use in High-Performance Bulk Heterojunction Polymer Cells. J. Am. Chem. Soc 2008, 130, 12828–12833. [Google Scholar]
- Koeppe, A; Bossart, O; Calzaferri, G; Sariciftci, NS. Advanced photon-harvesting concept for low-energy gap organic solar cells. Sol. Energ. Mater. Sol. Cell 2007, 91, 986–995. [Google Scholar]
- Shirakawa, H; Louis, EJ; MacDiarmid, AG; Chiang, CK; Heeger, AJ. Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun 1977, 16, 578–580. [Google Scholar]
- Thompson, BC; Frechet, JMJ. Polymer–Fullerene Composite Solar Cells. Angew. Chem. Int. Ed 2008, 47, 58–77. [Google Scholar]
- Guldi, DM; Gouloumis, A; Vázquez, P; Torres, T; Georgakilas, V; Prato, M. Nanoscale Organization of a Phthalocyanine-Fullerene System: Remarkable Stabilization of Charges in Photoactive 1-D Nanotubules. J. Am. Chem. Soc 2005, 127, 5811–5813. [Google Scholar]
- Jones, BA; Facchetti, A; Wasielewski, MR; Marks, TJ. Tuning Orbital Energetics in Arylene Diimide Semiconductors. Materials Design for Ambient Stability of n-Type Charge Transport. J. Am. Chem. Soc 2007, 129, 15259–15278. [Google Scholar]
- Prato, M. [60]Fullerene chemistry for materials science applications. J. Mater. Chem 1997, 7, 1097–1109. [Google Scholar]
- Anthony, JE. The Larger Acenes: Versatile Organic Semiconductors. Angew. Chem. Int. Ed 2008, 47, 452–483. [Google Scholar]
- Anthony, JE. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev 2006, 106, 5028–5048. [Google Scholar]
- Sim, JH; Kim, SJ; Yamada, K; Yokokura, S; Natori, I; Natori, S; Sato, H. Synthesis and characterization of polytriphenylamine containing ether bond in the main chain. Synth. Metals 2009, 159, 85–90. [Google Scholar]
- Bredas, JL; Chance, RR; Baughman, RH; Silbey, R. Ab initio effective Hamiltonian study of the electronic properties of conjugated polymers. J. Chem. Phys 1982, 76, 3673–3678. [Google Scholar]
- Young, JK; Moore, JS. Modern Acetylene Chemistry; Stang, PJ, Diederich, F, Eds.; VCH: Weinheim, Germany, 1995; pp. 415–442. [Google Scholar]
- Nielsen, MB; Diederich, F. Conjugated Oligoenynes Based on the Diethynylethene Unit. Chem. Rev 2005, 105, 1837–1867. [Google Scholar]
- Bunz, UHF. Poly(aryleneethynylene)s: Syntheses, Properties, Structures, and Applications. Chem. Rev 2000, 100, 1605–1644. [Google Scholar]
- Tang, BZ. Construction of Functional Polymers from Acetylenic Triple-Bond Building Blocks. Macromol. Chem. Phys 2008, 209, 1303–1307. [Google Scholar]
- Li, C; Li, Y. The Process of Functional Conjugated Organic Polymers Derived from Triple-Bond Building Blocks. Macromol. Chem. Phys 2008, 209, 1541–1552. [Google Scholar]
- Kivala, M; Diederich, F. Acetylene-Derived Strong Organic Acceptor for Planar and Nonplanar Push-Pull Chromophores. Acc. Chem. Res 2009, 42, 235–248. [Google Scholar]
- Siemsen, P; Livingston, RC; Diederich, F. Acetylenic Coupling: A Powerful Tool in Molecular Construction. Angew. Chem. Int. Ed 2000, 39, 2632–2657. [Google Scholar]
- Liu, J; Lam, JWY; Tang, BZ. Acetylenic Polymers: Syntheses, Structure, and Functions. Chem. Rev 2009, 109, 5799–5867. [Google Scholar]
- Sonogashira, K; Tohda, Y; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett 1975, 16, 4467–4470. [Google Scholar]
- Sonogashira, K. Development of Pd–Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem 2002, 653, 46–49. [Google Scholar]
- Negishi, EJ; Anastasia, L. Palladium-Catalyzed Alkynylation. Chem. Rev 2003, 103, 1979–2018. [Google Scholar]
- Chinchilla, R; Carmen Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev 2007, 107, 874–922. [Google Scholar]
- Alami, M; Ferri, F; Linstrumelle, G. An efficient palladium-catalyzed reaction of vinyl and aryl halides or triflates with terminal alkynes. Tetrahedron Lett 1993, 34, 6403–6406. [Google Scholar]
- Mori, A; Kawashima, J; Shimada, T; Suguro, M; Hirabayashi, K; Nishihara, Y. Non-Sonogashira-Type Palladium-Catalyzed Coupling Reactions of Terminal Alkynes Assisted by Silver(I) Oxide or Tetrabutylammonium Fluoride. Org. Lett 2000, 2, 2935–2937. [Google Scholar]
- Tykwinski, RR. Evolution in the Palladium-Catalyzed Cross-Coupling sp- and sp2-Hybridized Carbon Atoms. Angew. Chem. Int. Ed 2003, 42, 1566–1568. [Google Scholar]
- Monnier, F; Turtaut, F; Duroure, L; Taillefer, M. Copper-Catalyzed Sonogashira type Reactions Under Mild Palladium-Free Conditions. Org. Lett 2008, 10, 3203–3206. [Google Scholar]
- Liang, B; Dai, M; Chen, J; Yang, Z. Copper-Free Sonogashira Coupling Reaction with PdCl2 in Water under Aerobic Conditions. J. Org. Chem 2005, 70, 391–393. [Google Scholar]
- Kuang, YY; Chen, FE. Copper- and Phosphine-Free Sonogashira Coupling Reaction Catalyzed by Polyurea-Encapsulated Palladium (II). Helvetica Chimica. Acta 2009, 92, 897–902. [Google Scholar]
- Bohm, VPW; Herrmann, WA. Coordination Chemistry and Mechanisms of Metal-Catalyzed C-C Coupling Reactions, A Copper-Free Procedure for the Palladium-Catalyzed Sonogashira Reaction of Aryl Bromides with Terminal Alkynes at Room Temperature. Eur. J. Org. Chem 2000, 22, 3679–3681. [Google Scholar]
- Urgaonkar, S; Verkade, JG. Ligand-, Copper-, and Amine-Free Sonogashira Reaction of Aryl Iodides and Bromides with Terminal Alkynes. J. Org. Chem 2004, 69, 5752–5755. [Google Scholar]
- Yi, C; Hua, R. Efficient Copper-Free PdCl2(PCy3)2-Catalyzed Sonogashira Coupling of Aryl Chloride with Terminal Alkynes. J. Org. Chem 2006, 71, 2535–2537. [Google Scholar]
- de Souza, ROMA; Bittar, MS; Mendes, LVP; da Silva, CMF; da Silva, VT; Antunes, OAC. Copper-Free Sonogashira Reaction Using Gold Nanoparticles Supported on Ce2O3, Nb2O5 and SiO2 under Microwave Irradiation. Synlett 2008, 12, 1777–1780. [Google Scholar]
- Zhang, G. Easy Copper-, Ligand- and Amine-Free Sonogashira Coupling Reaction Catalyzed by Palladium on Carbon at Low Catalyst Loading and by Exposure to Air. Synlett 2005, 4, 619–622. [Google Scholar]
- Fulmer, DA; Shearouse, WC; Mendonza, ST; Mack, J. Solvent-free Sonogashira coupling reaction via high speed ball milling. Green Chem 2009, 11, 1821–1825. [Google Scholar]
- Diederich, F. Advanced opto-electronics materials by fullerene and acetylene scaffolding. Pure Appl. Chem 2005, 77, 1851–1864. [Google Scholar]
- Soares do Rego Barros, O; Favero, A; Nogueira, CW; Menezes, PH; Zeni, G. Palladium-catalyzed cross-coupling of 2-haloselenophene with with terminal alkyne in the absence of additive. Tetrahedron Lett 2006, 47, 2179–2182. [Google Scholar]
- Brizius, G; Pschirer, NG; Steffen, W; Stitzer, K; zur Loye, HC; Bunz, UHF. Alkyne Metathesis with Simple Catalyst Systems: Efficient Synthesis of Conjugated Polymers Containing Vinyl Groups in Main or Side Chain. J. Am. Chem. Soc 2000, 122, 12435–12440. [Google Scholar]
- Schrock, RR; Czekelius, C. Recent Advances in the Syntheses and Applications of Molybdenum and Tungsten Alkylidene and Alkylidyne Catalysts for the Metathesis of Alkenes and Alkynes. Adv. Synth. Catal 2007, 349, 55–77. [Google Scholar]
- Fürstner, A; Davies, PW. Alkyne metathesis. Chem. Commun 2005, 18, 2307–2320. [Google Scholar]
- Katz, TJ; McGinnis, J. The Mechanism of the Olefin Methathesis Reaction. J. Am. Chem. Soc 1975, 97, 1592–1594. [Google Scholar]
- Bunz, UHF. Poly(p-phenyleneethynylene)s by Alkyne Methathesis. Acc. Chem. Res 2001, 34, 998–1010. [Google Scholar]
- Fürstner, AF; Mathes, C; Lehmann, CW. Mo[N(t-Bu)(Ar)]3 Complexes As Catalyst Precursors: In Situ Activation and Application to Metathesis Reactions of Alkynes and Diynes. J. Am. Chem. Soc 1999, 121, 9453–9454. [Google Scholar]
- Fürstner, AF; Mathes, C; Lehmann, C. Alkyne Metathesis: Development of a Novel Molybdenum-Based Catalyst System and Its Application to the Total Synthesis of Epothilone A and C. Chem. Eur. J 2001, 7, 5299–5317. [Google Scholar]
- Jain, V; Rajbongshi, BK; Mallajosyula, TA; Bhattacharjya, G; Iyer, SSK; Ramanathan, G. Photovoltaic effect in single-layer organic solar cell devices fabricated with two new imidazolin-5-one molecules. Sol. Energ. Mater. Sol. Cell 2008, 92, 1043–1046. [Google Scholar]
- Fang, Y; Chen, SA; Chu, ML. Effect on the side chain length on rectification and photovoltaic characteristics of poly(3-alkylthiophene) Schottky barriers. Synth. Met 1992, 52, 261–272. [Google Scholar]
- Glenis, S; Horowitz, G; Tourillon, G; Garnier, F. Electrochemically grown polythiophene and poly(3-methylthiophene) organic photovoltaic cells. Thin Solid Films 1984, 111, 93–103. [Google Scholar]
- Coakley, KM; McGehee, MD. Conjugated polymer photovoltaic cells. Chem. Mater 2004, 16, 4533–4542. [Google Scholar]
- Janssen, RAJ; Hummelen, JC; Sariciftci, NS. Polymer fullerene bulk heterojunction solar cells. MRS Bull 2005, 30, 33–36. [Google Scholar]
- Forrest, SR. The limits to organic photovoltaic cell efficiency. MRS Bull 2005, 30, 28–32. [Google Scholar]
- Peumans, P; Yakimov, A; Forrest, SR. Small molecular weight organic thin-film photodetectors and solar cells. J. Appl. Phys 2003, 93, 3693–3723. [Google Scholar]
- Coakley, KM; Liu, YX; Goh, C; McGehee, MD. Ordered organic-inorganic bulk heterojunction photovoltaic cells. MRS Bull 2005, 30, 37–40. [Google Scholar]
- Milliron, DJ; Gur, I; Alivisatos, AP. Hybrid organic-nanocrystal solar cells. MRS Bull 2005, 30, 41–44. [Google Scholar]
- Schmidt-Mende, L; Bach, U; Humphry-Baker, R; Horiuchi, T; Miura, H; Ito, S; Uchida, S; Grätzel, M. Organic dye for highly efficient solid-state dye sensitized solar cells. Adv. Mater 2005, 17, 813–815. [Google Scholar]
- Grätzel, M. Perspectives for dye-sensitized nanocrystalline solar cells. Prog. Photovolt: Res. Appl 2000, 8, 171–185. [Google Scholar]
- Liang, Y; Xu, Z; Xia, J; Tsai, ST; Wu, Y; Li, G; Ray, C; Yu, L. For the Bright Future–Bulk Heterojunction Polymer Solar Cells with Power Conversion Efficiency of 7.4%. Adv Mater 2010, 22. [Google Scholar]
- Brabec, CJ; Cravino, A; Meissner, D; Sariciftci, NS; Fromherz, T; Rispens, MT; Sanchez, L; Hummelen, JC. Origin of the open circuit voltage of plastic solar cells. Adv. Funct. Mater 2001, 11, 374–380. [Google Scholar]
- Soci, C; Hwang, IW; Moses, D; Zhu, Z; Waller, D; Guadiana, R; Brabec, CJ; Heeger, AJ. Photoconductivity of a Low-Bandgap Conjugated Polymer. Adv. Funct. Mater 2007, 17, 632–636. [Google Scholar]
- Coakley, KM; McGehee, MD. Conjugated Polymer Photovoltaic Cells. Chem. Mater 2004, 16, 4533–4542. [Google Scholar]
- Gunes, S; Neugebauer, H; Sariciftci, NS. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev 2007, 107, 1324–1338. [Google Scholar]
- Rand, BP; Genoe, J; Heremans, P; Poortmans, J. Solar cells utilizing small molecular weight organic semiconductors. Prog. Photovolt.: Res. Appl 2007, 15, 659–676. [Google Scholar]
- Matthew Lloyd, T; Anthony, JE; Malliaras, GG. Photovoltaics from soluble small molecules. Mater. Today 2007, 10, 34–41. [Google Scholar]
- Walker, B; Tamayo, AB; Dang, XD; Zalar, P; Seo, HJ; Garcia, A; Tantiwiwat, M; Nguyen, TQ. Nanoscale Phase Separation and High Photovoltaic Efficiency in Solution-Processed, Small-Molecule Bulk Heterojunction Solar Cells. Adv. Funct. Mater 2009, 19, 1–7. [Google Scholar]
- Lloyd, MT; Mayer, AC; Subramanian, S; Mourey, DA; Herman, DJ; Bapat, AV; Anthony, JE; Malliaras, GG. Efficient Solution-Processed Photovoltaic Cells Based on an Anthradithiophene/Fullerene Blend. J. Am. Chem. Soc 2007, 129, 9144–9149. [Google Scholar]
- Valentini, L; Marrocchi, A; Seri, M; Mengoni, F; Meloni, F; Taticchi, A; Kenny, JM. [2.2]Paracyclophane-based molecular systems for the development of organic solar cells. Thin Solid Films 2008, 516, 7193–7198. [Google Scholar]
- Valentini, L; Bagnis, D; Marrocchi, A; Seri, M; Taticchi, A; Kenny, JM. Novel Anthracene-Core Molecule for the Development of Efficient PCBM-Based Solar Cells. Chem. Mater 2008, 20, 32–34. [Google Scholar]
- Marrocchi, A; Silvestri, F; Seri, M; Facchetti, A; Taticchi, A; Marks, TJ. Conjugated anthracene derivatives as donor materials for bulk heterojunction solar cells: Olefinic versus acetylenic spacers. Chem Commun 2009, 1380–1382. [Google Scholar]
- Hopf, H. Recent Uses of [2.2]Paracyclophanes in Polymer Chemistry and Material Science. Angew. Chem. Int. Ed 2008, 47, 9808–9812. [Google Scholar]
- Umnov, AG; Korovyanko, OJ. Photovoltaic effect in poly-dioctyl-phenylene-ethynylene-C60 cells upon donor and acceptor excitation. Appl. Phys. Lett 2005, 87, 113506. [Google Scholar]
- Cremer, J; Bäuerle, P; Wienk, MM; Janssen, RAJ. High Open-Circuit Voltage Poly(ethynylene bithienylene): Fullerene Solar Cells. Chem. Mater 2006, 18, 5832–5834. [Google Scholar]
- Liu, Q; Mao, J; Liu, Z; Zhang, N; Wang, Y; Yang, L; Yin, S; Chen, Y. A photovoltaic device based on a poly(phenylene ethynylene)/SWNT composite active layer. Nanotechnology 2008, 19, 115601-1–115601-5. [Google Scholar]
- Egbe, DAM; Carbonnier, B; Birckner, E; Grummt, UH. Arylene-ethynylene/arylene-vinylene copolymers: Synthesis and structure-property relationships. Prog. Polym. Sci 2009, 34, 1023–1067. [Google Scholar]
- Schenning, APHJ; Tsipis, AC; Meskers, SCJ; Beljonne, D; Meijer, EW; Bredas, JL. Electronic Structure and Optical Properties of Mixed Phenylene Vinylene/Phenylene Ethynylene Conjugated Oligomers. Chem. Mater 2002, 14, 1362–1368. [Google Scholar]
- Swager, TM; Gil, CJ; Wrighton, MS. Fluorescence Studies of Poly(p-phenyleneethyny1ene)s: The Effect of Anthracene Substitution. J. Phys. Chem 1995, 99, 4886–4893. [Google Scholar]
- Chu, Q; Pang, Y; Ding, L; Karasz, FE. Green-Emitting PPE–PPV Hybrid Polymers: Efficient Energy Transfer across the m-Phenylene Bridge. Macromolecules 2003, 36, 3848–3853. [Google Scholar]
- Tong, M; Sheng, CX; Yang, C; Vardeny, ZV; Pang, Y. Photoexcitation dynamics and laser action in solutions and films of PPE-PPV copolymer. Phys Rev B 2004, 69, 155211/1–155211/8. [Google Scholar]
- Hoppe, H; Egbe, DAM; Mühlbacher, D; Sariciftci, NS. Photovoltaic action of conjugated polymer/fullerene bulk heterojunction solar cells using novel PPE-PPV copolymers. J. Mater. Chem 2004, 14, 3462–3467. [Google Scholar]
- Egbe, DAM; Wild, A; Birckner, E; Grummt, UW; Schubert, US. Anthracene- and Thiophene-Containing Poly(p-arylene-ethynylene)/poly(p-arylene-vinylene)s: Towards Optimized Structures for Photovoltaic Applications. Macromol. Symp 2008, 268, 25–27. [Google Scholar]
- Egbe, DAM; Nguyen, LH; Schmidtke, K; Wild, A; Sieber, C; Guenes, S; Sariciftci, NS. Combined Effects of Conjugation Pattern and Alkoxy Side Chains on the Photovoltaic Properties of Thiophene-Containing PPE-PPVs. J. Pol. Sci. A: Polym. Chem 2009, 47, 2243–2261. [Google Scholar]
- Rathgeber, S; de Toledo, DB; Birckner, E; Hoppe, H; Egbe, DAM. Intercorrelation between Structural Ordering and Emission Properties in Photoconducting Polymers. Macromolecules 2010, 43, 306–315. [Google Scholar]
- Egbe, DAM; Türk, S; Rathgeber, S; Kühnlenz, F; Jadhav, R; Wild, A; Birckner, E; Adam, G; Pivrikas, A; Cimrova, V; Knör, G; Sariciftci, NS; Hoppe, H. Anthracene Based Conjugated Polymers: Correlation between π-π-Stacking Ability, Photophysycal Properties, Charge Carrier Mobility, and Photovoltaic Performance. Macromolecules 2010, 43, 1261–1269. [Google Scholar]
- Jadhav, R; Türk, S; Kühnlenz, F; Cimrova, V; Rathgeber, S; Egbe, DAM; Hoppe, H. Anthracene-containing PPE-PPV copolymers: Effect of side-chain nature and length on photophysical and photovoltaic properties. Phys. Status Solidi A 2009, 206, 2695–2699. [Google Scholar]
- Al-Ibrahim, M; Konkina, A; Rotha, HK; Egbe, DAM; Klemmb, E; Zhokhavetsc, U; Gobschc, G; Sensfussa, S. Phenylene-ethynylene/phenylene-vinylene hybrid polymers: Optical and electrochemical characterization, comparison with poly[2-methoxy-5-(3V,7V-dimethyloctyloxy)-1,4-phenylene vinylene] and application in flexible polymer solar cells. Thin Solid Films 2005, 474, 201–210. [Google Scholar]
- Miteva, T; Palmer, L; Kloppenburg, L; Neher, D; Bunz, UHF. Interplay of Thermochromicity and Liquid Crystalline Behavior in Poly(p-phenylene ethynylene)s: π-π Interactions or Planarization of the Conjugated Backbone? Macromolecules 2000, 33, 652–654. [Google Scholar]
- Egbe, DAM; Nguyen, LH; Hoppe, H; Mühlbacher, D; Sariciftci, NS. Side Chain Influence on Electrochemical and Photovoltaic Properties of Yne-Containing Poly(phenylene vinylene)s. Macromol. Rapid Commun 2005, 26, 1389–1394. [Google Scholar]
- Egbe, DAM; Ulbricht, C; Orgis, T; Carbonnier, B; Kietzke, T; Peip, M; Metzner, M; Gericke, M; Birckner, E; Pakula, T; Neher, D; Grummt, UV. Odd-Even Effects and the Influence of Length and Specific Positioning of Alkoxy Side Chains on the Optical Properties of PPE-PPV Polymers. Chem. Mater 2005, 17, 6022–6032. [Google Scholar]
- Kietzke, T; Shin, RYC; Egbe, DAM; Chen, ZK; Sellinger, A. Effect of Annealing on the Characteristics of Organic Solar Cells: Polymer Blends with a 2-Vinyl-4,5-dicyanoimidazole Derivative. Macromolecules 2007, 40, 4424–4428. [Google Scholar]
- McGehee, MD. Organic solar cells: Overcoming recombination. Nat. Photon 2009, 3, 250–252. [Google Scholar]
- Park, SH; Roy, A; Beaupré, S; Cho, S; Coates, N; Moon, JS; Moses, D; Leclerc, M; Lee, K; Heeger, AJ. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photon 2009, 3, 297–303. [Google Scholar]
- Egbe, DAM; Nguyen, LH; Carbonnier, B; Mühlbacher, D; Sariciftci, NS. Thiophene-containing poly(arylene–ethynylene)-alt-poly(arylene–vinylene)s: Synthesis, characterisation and optical properties. Polymer 2005, 46, 9585–9595. [Google Scholar]
- Egbe, DAM; Nguyen, LH; Mühlbacher, D; Hoppe, H; Schmidtke, K; Sariciftci, NS. Photophysical, electrochemical and photovoltaic properties of thiophene-containing arylene-ethynylene/arylene-vinylene polymers. Thin Solid Films 2006, 511–512, 486–488. [Google Scholar]
- Lu, S; Yang, M; Luo, J; Cao, Y. Photovoltaic devices based on a novel poly(phenylene ethynylene) derivative. Synth. Metals 2004, 140, 199–202. [Google Scholar]
- Lu, S; Yang, M; Luo, J; Cao, Y; Bai, F. Novel Alternating Dioctyloxyphenylene Vinylene-Benzothiadiazole Copolymer: Synthesis and Photovoltaic Performance. Macromol. Chem. Phys 2005, 206, 664–671. [Google Scholar]
- Hou, Y; Chen, Y; Liu, Q; Yang, M; Wan, X; Yin, S; Yu, A. A Novel Tetrathiafulvalene-(TTF-) Fused Poly(aryleneethynylene) with an Acceptor Main Chain and Donor Side Chains: Intramolecular Charge Transfer (CT), Stacking Structure, and Photovoltaic Property. Macromolecules 2008, 41, 3114–3119. [Google Scholar]
- Huang, X; Zhu, C; Li, W; Guo, Y; Zhang, S; Zhan, X; Liu, Y; Bo, Z. Porphyrin-dithienothiophene π-conjugated copolymers: synthesis and their applications in field-effect transistors and solar cells. Macromolecules 2008, 41, 6895–6902. [Google Scholar]
- Egbe, DAM; Kietzke, T; Carbonnier, B; Mühlbacher, D; Hörhold, H-H; Neher, D; Pakula, T. Synthesis, Characterization, and Photophysical, Electrochemical, Electroluminescent, and Photovoltaic Properties of Yne-Containing CN-PPVs. Macromolecules 2004, 37, 8863–8873. [Google Scholar]
- Kietzke, T; Egbe, DAM; Hans-Hörhold, H-H; Neher, D. Comparative Study of M3EH-PPV-Based Bilayer Photovoltaic Devices. Macromolecules 2006, 39, 4018–4022. [Google Scholar]
- Ashraf, RS; Shahid, M; Klemm, E; Al-Ibrahim, M; Sensfuss, S. Thienopyrazine-Based Low-Bandgap Poly(heteroaryleneethynylene)s for Photovoltaic Devices. Macromol. Rapid Commun 2006, 27, 1454–1459. [Google Scholar]
- Schull, TL; Kushmerick, JG; Patterson, CH; George, C; Moore, MH; Pollack, SK; Shashidhar, R. Ligand effects on charge transport in platinum(II) acetylides. J. Am. Chem. Soc 2003, 125, 3202–3203. [Google Scholar]
- Holliday, BJ; Swager, TM. Conducting metallopolymers: the roles of molecular architecture and redox matching. Chem Commun 2005, 23–36. [Google Scholar]
- Köhler, A; Wittmann, HF; Friend, RH; Khan, MS; Lewis, J. Enhanced photocurrent response in photocells made with platinum- poly-yne/C60 blends by photoinduced electron transfer. Synth. Metals 1996, 77, 147–150. [Google Scholar]
- Dennler, G; Scharber, MC; Brabec, CJ. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv Mater 2009, 21, 1323–1338. [Google Scholar]
- Xue, J; Uchida, S; Rand, BP; Forrest, SR. 4.2% Efficient organic photovoltaic cells with low series resistances. Appl. Phys. Lett 2004, 84, 3013–3015. [Google Scholar]
- Robertson, N. Optimizing dyes for dye-sensitized solar cells. Angew. Chem. Int. Ed 2006, 45, 2338–2345. [Google Scholar]
- Nguyen, P; Gomez-Elipe, P; Manners, I. Organometallic polymers with transition metals in the main chain. Chem. Rev 1999, 99, 1515–1548. [Google Scholar]
- Williams, KA; Boydston, AJ; Bielawski, CW. Main-chain organometallic polymers: Synthetic strategies, applications, and perspectives. Chem. Soc. Rev 2007, 36, 729–744. [Google Scholar]
- Köhler, A; Wittmann, HF; Friend, RH; Khan, MS; Lewis, J. The Photovoltaic effect in a platinum poly-yne. Synth. Metals 1994, 67, 245–249. [Google Scholar]
- Chawdhury, N; Köhler, A; Friend, RH; Wong, WY; Lewis, J; Younus, M; Raithby, PR; Corcoran, TC; Al-Mandhary, MRA; Khan, MS. Evolution of lowest singlet and triplet excited states with number of thienyl rings in platinum poly-ynes. J. Chem. Phys 1999, 110, 4963–4970. [Google Scholar]
- Guo, F; Kim, YG; Reynolds, JR; Schanze, KS. Platinum–Acetylide polymer based solar cells: Involvement of the triplet state for energy conversion. Chem. Commun 2006, 17, 1887–1889. [Google Scholar]
- Younus, M; Köhler, A; Cron, S; Chawdhury, N; Al-Mandhary, MRA; Khan, MS; Lewis, J; Long, NJ; Friend, RH; Raithby, PR. Synthesis, electrochemistry, and spectroscopy of blue platinum(II) polyynes and diynes. Angew. Chem. Int. Ed 1998, 37, 3036–3039. [Google Scholar]
- Wong, WY; Wang, XZ; He, Z; Chan, KK; Djurisîć, AB; Cheung, KY; Yip, CT; Ng, AMG; Xi, YY; Mak, CSK; Chan, WK. Tuning the Absorption, Charge Transport Properties, and Solar Cell Efficiency with the Number of Thienyl Rings in Platinum-Containing Poly(aryleneethynylene)s. J. Am. Chem. Soc 2007, 129, 14372–14380. [Google Scholar]
- Liu, L; Ho, CL; Wong, WY; Cheung, KY; Fung, MK; Lam, WT; Djurisîć, AB; Chan, WK. Effect of Oligothienyl Chain Length on Tuning the Solar Cell Performance in Fluorene-Based Polyplatinynes. Adv. Funct. Mater 2008, 18, 2824–2833. [Google Scholar]
- Wong, WY; Wang, XZ; He, Z; Djurisîć, AB; Yip, CT; Cheung, KY; Wang, H; Mak, CSK; Chan, WK. Metallated conjugated polymers as a new avenue towards high-efficiency polymer solar cells. Nat. Mater 2007, 6, 521. [Google Scholar]
- Gilot, J; Wienk, MM; Janssen, RAJ. On the efficiency of polymer solar cells. Nat. Mater 2007, 6, 704. [Google Scholar]
- Wong, WY; Wang, XZ; He, Z; Djurisic, AB; Yip, CT; Cheung, KY; Wang, H; Mak, CSK; Chan, WK. On the efficiency of polymer solar cells. Nat. Mater 2007, 6, 704–705. [Google Scholar]
- Baek, NS; Hau, SK; Yip, HL; Acton, O; Chen, KS; Jen, AKY. High Performance Amorphous Metallated π-Conjugated Polymers for Field-Effect Transistors and Polymer Solar Cells. Chem. Mater 2008, 20, 5734–5736. [Google Scholar]
- Mei, J; Ogawa, K; Kim, YG; Heston, NC; Arenas, DJ; Nasrollahi, Z; McCarley, TD; Tanner, DB; Reynolds, JR; Schanze, KS. Low-Band-Gap Platinum Acetylide Polymers as Active Materials for Organic Solar Cells. Appl. Mater. Interf 2009, 1, 150–161. [Google Scholar]
- Wong, WY; Wang, X; Zhang, HL; Cheung, KY; Djurišić, AB; Chan, WK. Synthesis, characterization and photovoltaic properties of a low bandgap platinum(II) polyyne functionalized with a 3,4-ethylenedioxythiophene-benzothiadiazole hybrid spacer. J. Organomet. Chem 2008, 693, 3603–3612. [Google Scholar]
- Wong, WY. Metallopolyyne Polymers as New Functional Materials for Photovoltaic and Solar Cell Applications. Macromol. Chem. Phys 2008, 209, 14–24. [Google Scholar]
- Wu, PT; Bull, T; Kim, FS; Luscombe, CK; Jenekhe, SA. Organometallic Donor-Acceptor Conjugated Polymer Semiconductors: Tunable Optical, Electrochemical, Charge Transport, and Photovoltaic Properties. Macromolecules 2009, 42, 671–681. [Google Scholar]
- Roncali, J. Linear π-conjugated systems derivatized with C60-fullerene as molecular heterojunctions for organic photovoltaics. Chem. Soc. Rev 2005, 34, 483–495. [Google Scholar]
- Segura, JL; Martín, N; Guldi, D. Materials for organic solar cells: the C60/π-conjugated oligomer approach. Chem. Soc. Rev 2005, 34, 31–47. [Google Scholar]
- Nierengarten, JF; Gu, T; Aernouts, T; Geens, W; Poortmans, J; Hadziioannou, G; Tsamouras, D. Fullerene–Oligophenylene ethynylene conjugates: Relationships between charge-carrier mobility, photovoltaic characteristics and chemical structure. Appl. Phys. A 2004, 79, 47–49. [Google Scholar]
- Gu, T; Tsamoursa, D; Melzer, C; Krasnikov, V; Gisselbrecht, JP; Gross, M; Hadziioannou, G; Nierengarten, JF. Photovoltaic Devices from Fullerene-Oligophenylene-ethynylene Conjugates. Chem. Phys Chem 2002, 3, 124–127. [Google Scholar]
- Gu, T; Nierengarten, JF. Synthesis of fullerene–oligophenylene ethynylene hybrids. Tetrahedron Lett 2001, 42, 3175–3178. [Google Scholar]
- Atienza, CM; Fernández, G; Sánchez, L; Martín, N; Dantas, IS; Wienk, MM; Janssen, RAJ; Aminur Rahmanc, GM; Guldi, D. Light harvesting tetrafullerene nanoarray for organic solar cells. Chem. Commun 2006, 5, 514–516. [Google Scholar]
- Fernández, G; Sánchez, L; Veldman, D; Wienk, MM; Atienza, C; Guldi, D; Janssen, RAJ; Martín, N. Tetrafullerene Conjugates for All-Organic Photovoltaics. J. Org. Chem 2008, 73, 3189–3196. [Google Scholar]
- Guo, F; Ogawa, K; Kim, YG; Danilov, EO; Castellano, FN; Reynolds, JR; Schanze, KS. A fulleropyrrolidine end-capped platinum-acetylide triad: the mechanism of photoinduced charge transfer in organometallic photovoltaic cells. Phys. Chem. Chem. Phys 2007, 9, 2724–2734. [Google Scholar]
- Ramos, AM; Rispens, MT; van Duren, JKJ; Hummelen, JC; Janssen, RAJ. Photoinduced Electron Transfer and Photovoltaic Devices of a Conjugated Polymer with Pendant Fullerenes. J. Am. Chem. Soc 2001, 123, 6714–6715. [Google Scholar]
- Mwaura, JK; Pinto, MR; Witker, D; Ananthakrishnan, N; Schanze, KS; Reynolds, JR. Photovoltaic Cells Based on Sequentially Adsorbed Multilayers of Conjugated Poly(p-phenylene ethynylene)s and a Water-Soluble Fullerene Derivative. Langmuir 2005, 21, 10119–10126. [Google Scholar]
- Tang, CW. Two Layer Organic Photovoltaic Cell. Appl. Phys. Lett 1986, 48, 183–185. [Google Scholar]

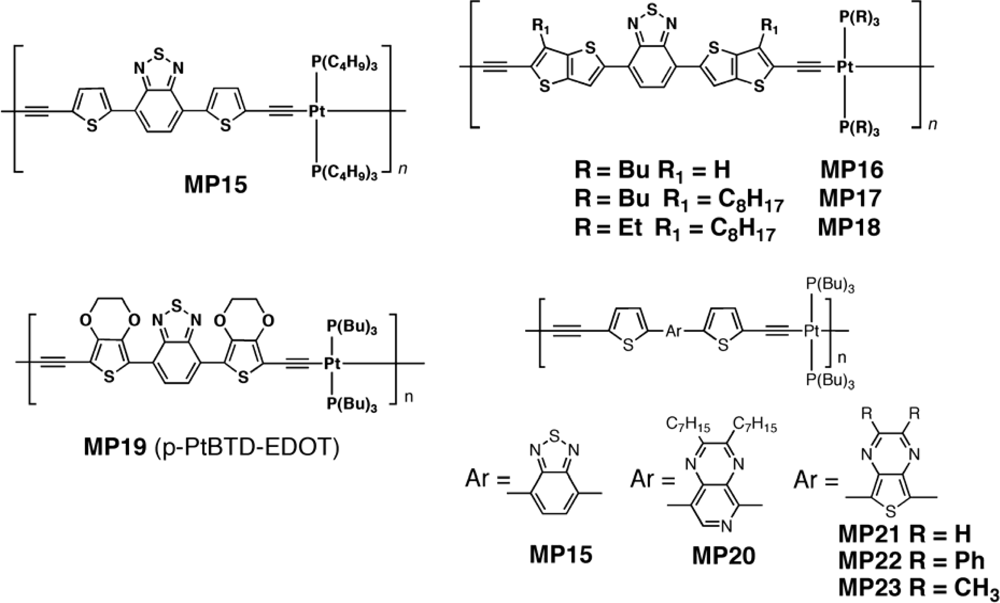
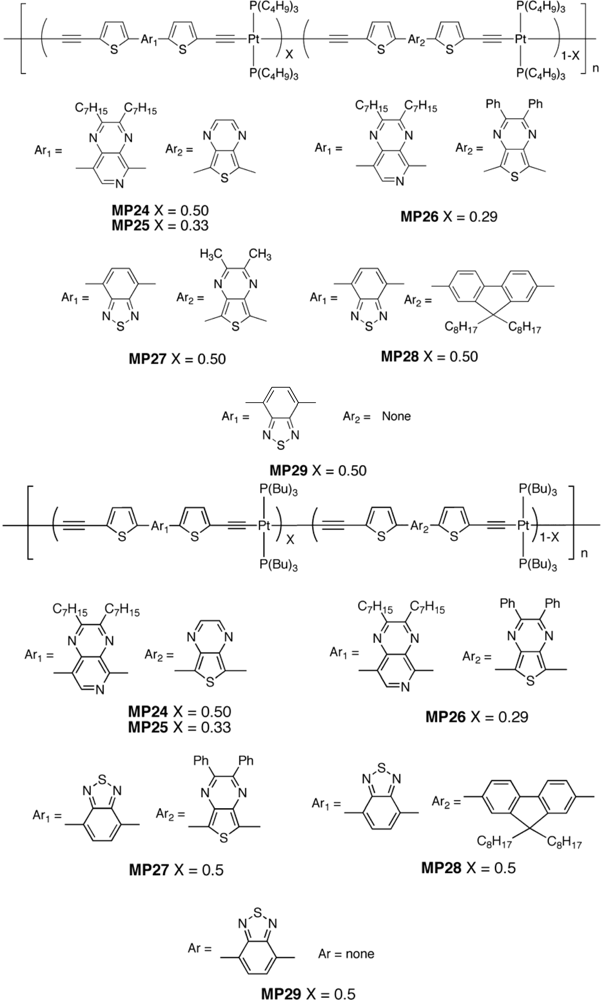
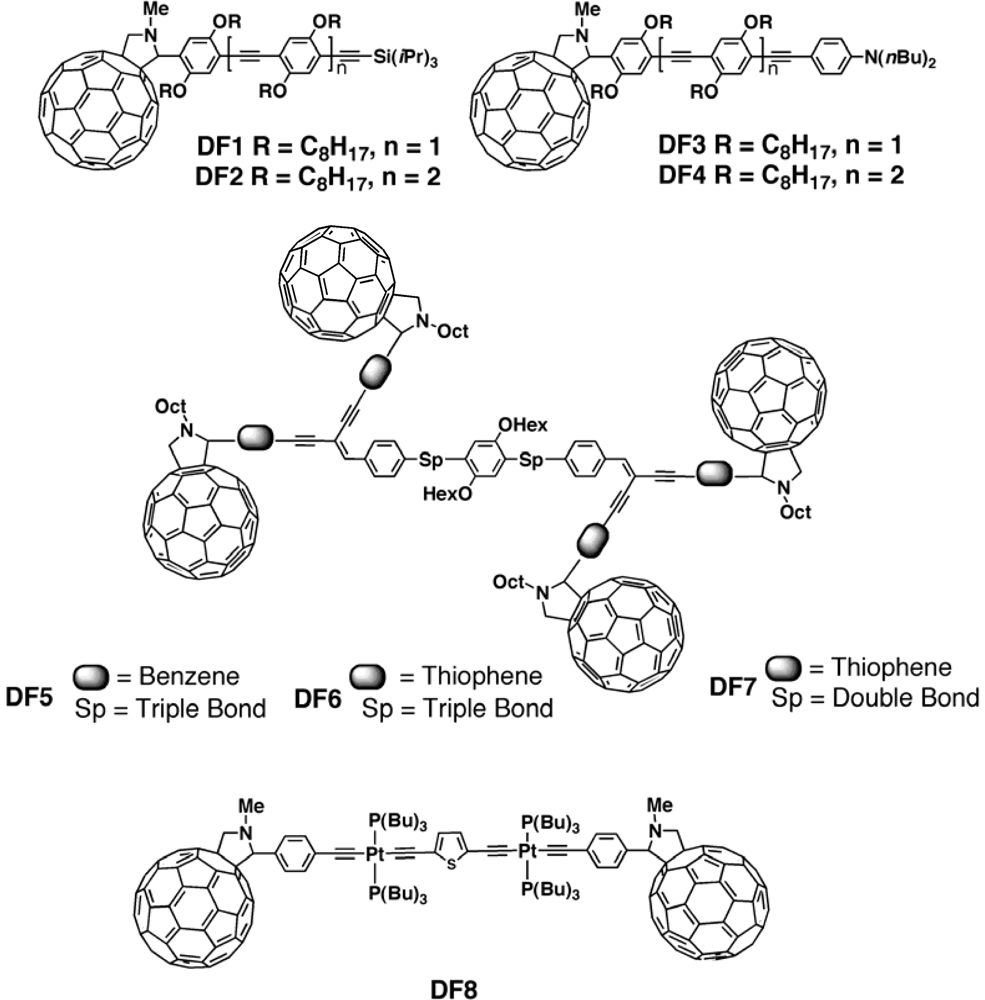
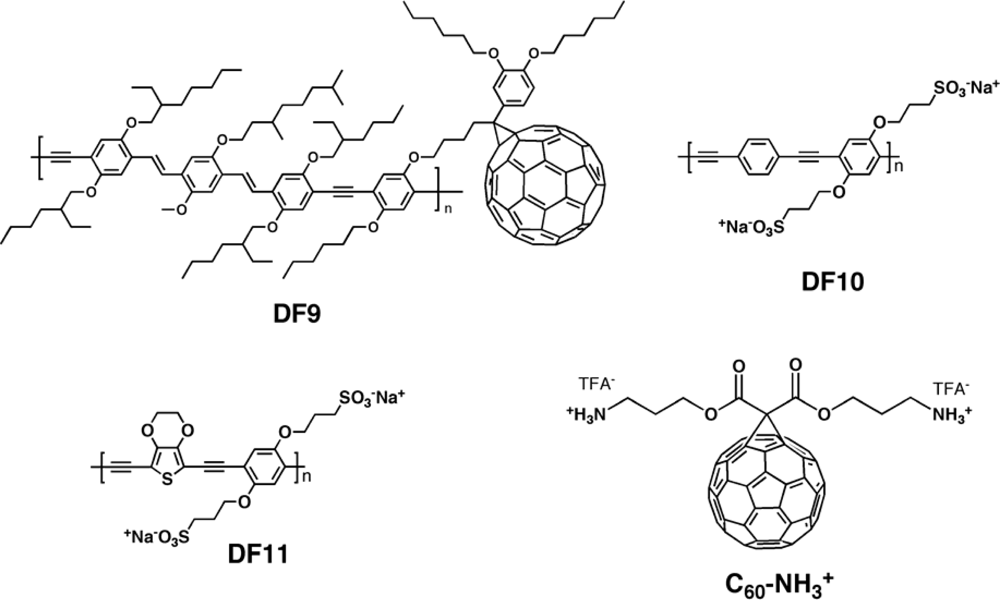
| Polymer Blenda | Thickness (nm) | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| MP15 : PC71BM | 75 | 0.77 | 9.65 | 32 | 2.41 |
| MP20 : PC71BM | 80 | 0.66 | 2.99 | 34 | 0.68 |
| MP21 : PC71BM | 65 | 0.52 | 2.71 | 26 | 0.36 |
| MP22 : PC71BM | 50–70 | 0.39 | 0.25 | 17 | 0.016 |
| MP23 : PC71BM | 50–70 | 0.53 | 2.14 | 28 | 0.32 |
| MP24 : PC61BM | 60 | 0.5 | 1.39 | 23 | 0.16 |
| MP25 : PC61BM | 60 | 0.5 | 0.99 | 23 | 0.11 |
| MP26 : PC61BM | 50–70 | 0.32 | 0.17 | 18 | 0.009 |
| MP27 : PC71BM | 50–70 | 0.52 | 0.86 | 25 | 0.11 |
| MP28 : PC71BM | 50–70 | 0.64 | 2.35 | 20 | 0.31 |
| MP29 : PC71BM | 50–70 | 0.68 | 4.21 | 25 | 0.71 |
| Blend | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| S6:PCBM [76] | 0.96 | 2.6 | 45 | 1.17 |
| P9:PCBM [93] | 0.79 | 7.14 | 55.65 | 3.14 |
| MP14:PCBM [124] | 0.89 | 7.56 | 43 | 2.88 |
| MP15:PCBM[125] | 0.82 | 15.43 | 39 | 4.93 |
| DF10:PCBM [142] | 0.26 | 0.50 | 31 | 0.041 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Silvestri, F.; Marrocchi, A. Acetylene-Based Materials in Organic Photovoltaics. Int. J. Mol. Sci. 2010, 11, 1471-1508. https://doi.org/10.3390/ijms11041471
Silvestri F, Marrocchi A. Acetylene-Based Materials in Organic Photovoltaics. International Journal of Molecular Sciences. 2010; 11(4):1471-1508. https://doi.org/10.3390/ijms11041471
Chicago/Turabian StyleSilvestri, Fabio, and Assunta Marrocchi. 2010. "Acetylene-Based Materials in Organic Photovoltaics" International Journal of Molecular Sciences 11, no. 4: 1471-1508. https://doi.org/10.3390/ijms11041471