Antioxidative Peptides Derived from Enzyme Hydrolysis of Bone Collagen after Microwave Assisted Acid Pre-Treatment and Nitrogen Protection
Abstract
:1. Introduction
2. Results and Discussions
2.1. Effect of Microwave Assisted Acid Pre-Treatment on Bone Collagen
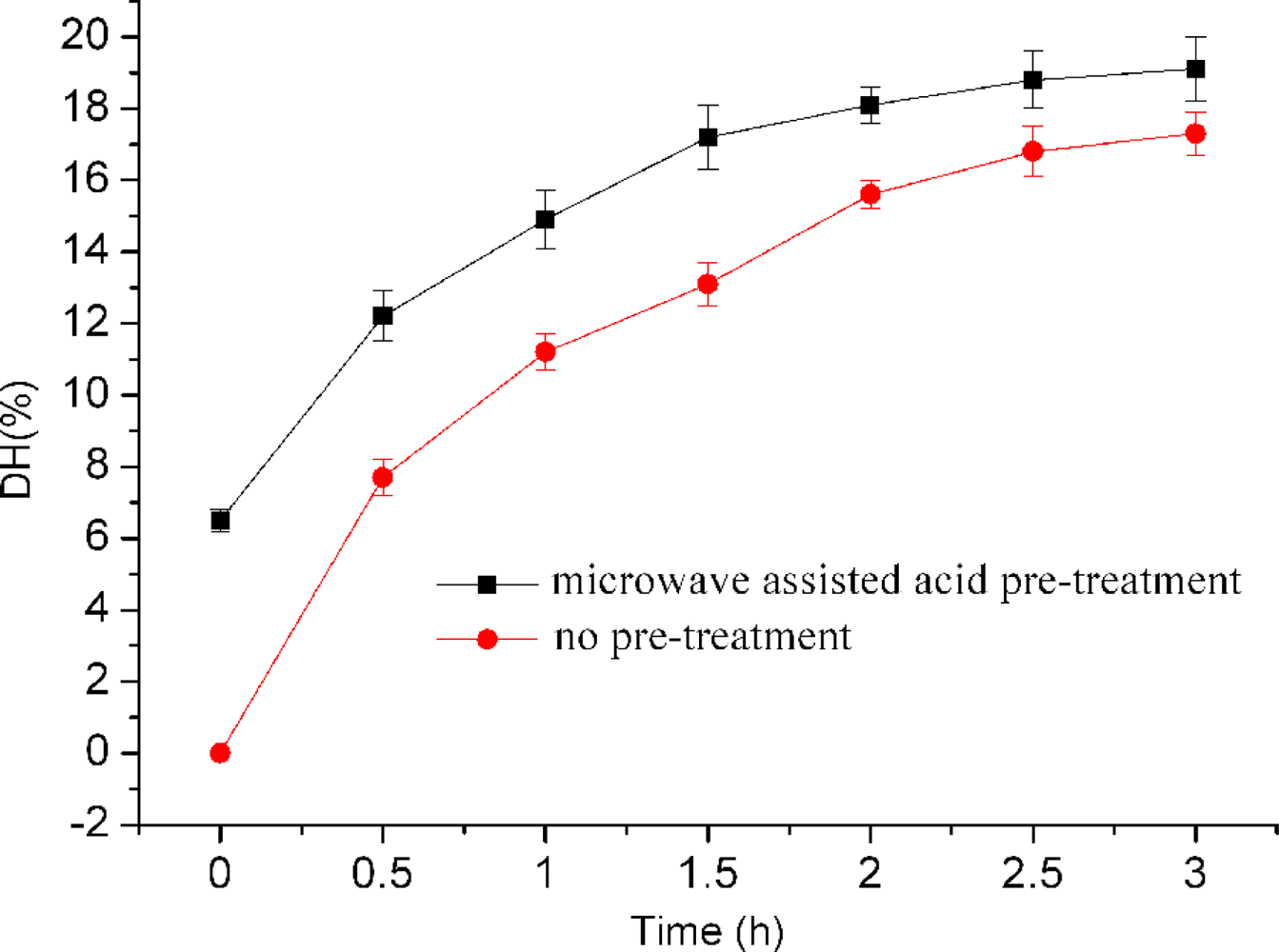
2.2. Effect of Microwave Assisted Acid Pre-Treatment on Enzyme Digestion of Bone Collagen
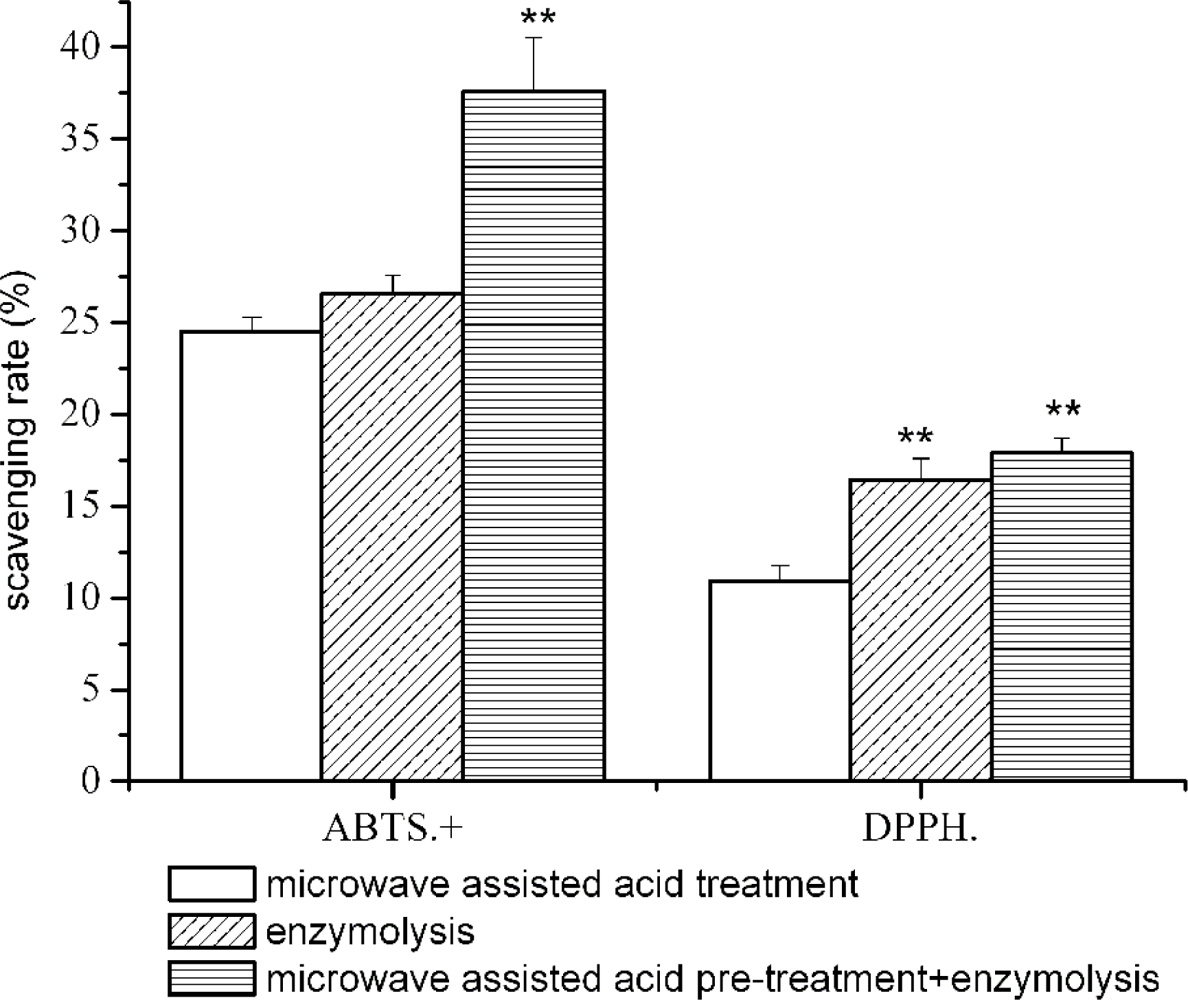
2.3. Effect of Microwave Assisted Acid Pre-treatment on the Antioxidant Activity
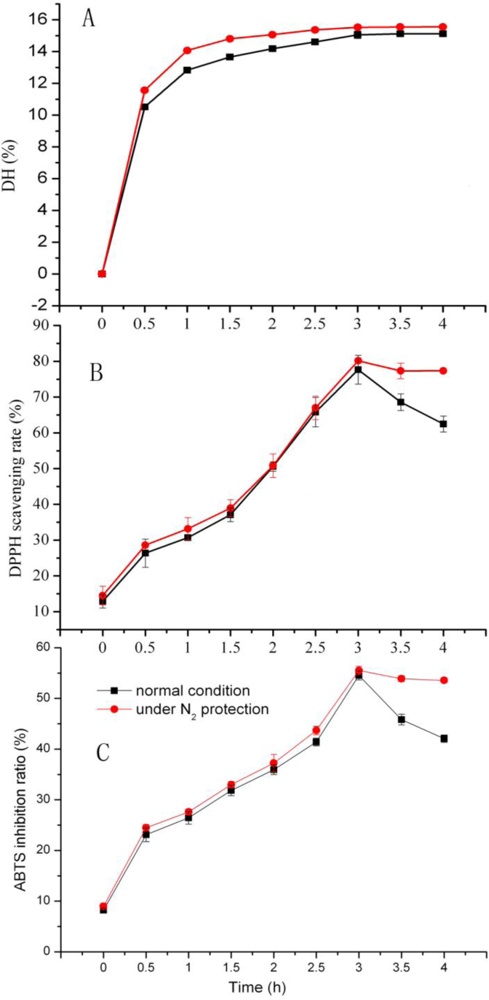
2.4. Effect of Nitrogen Protection on DH and Antioxidation Activity
3. Experimental
3.1. Materials
3.2. Preparation of Collagen Peptides
3.2.1. Microwave Assisted Acid Pre-treatment of Bone Collagen
3.2.2. Protease Hydrolysis of Pre-Treatment of Bone Collagen
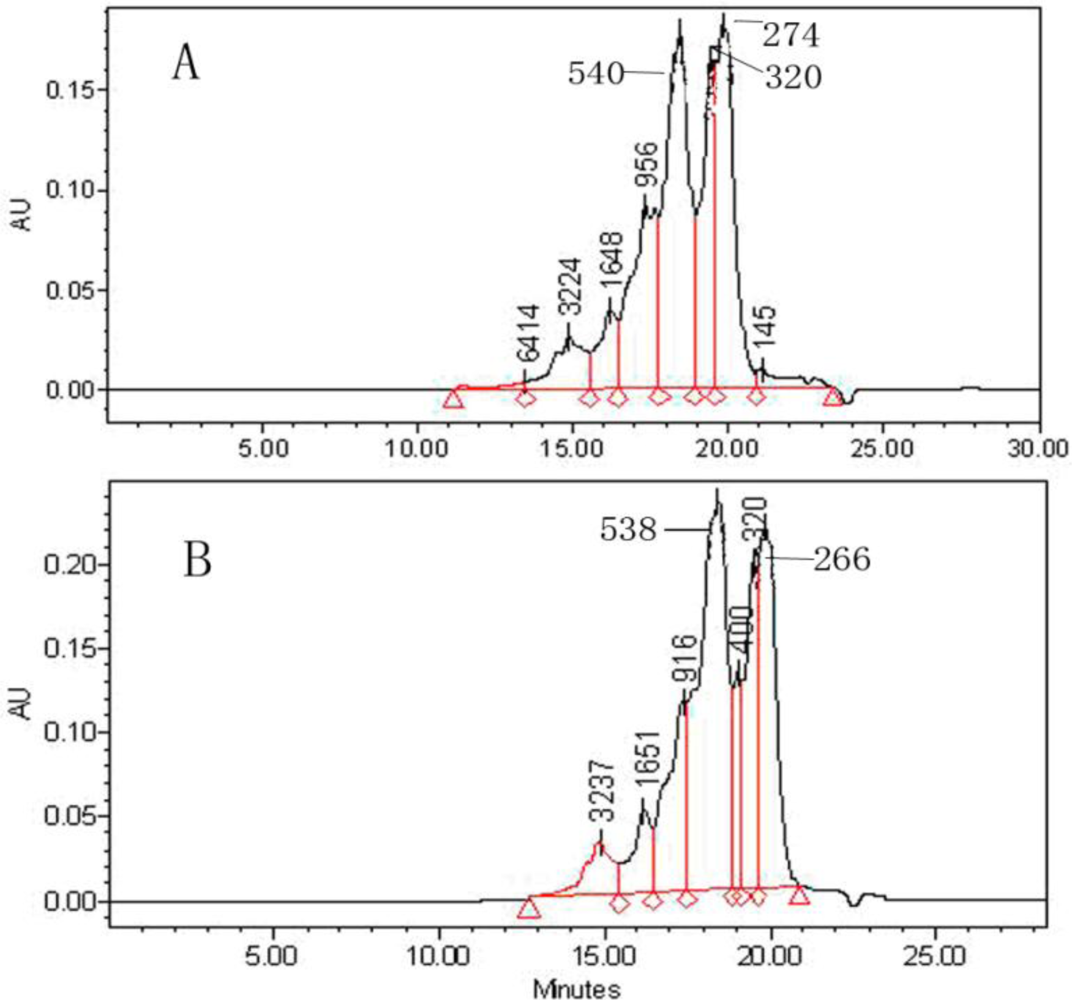
3.3. Determination of Molecular Weight Distribution (MW)
3.4. DPPH Radicals Scavenging Activity Assay
3.5. ABTS• Scavenging Activity Assay
3.6. Statistical Analysis
4. Conclusion
Acknowledgments
References
- Fratzl, P. Collagen: Structure and Mechanics; Springer: New York, NY, USA, 2008. [Google Scholar]
- Buehler, MJ. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. Proc. Nat. Acad. Sci. USA 2006, 103, 12285–12290. [Google Scholar]
- Iwai, K; Hasegawa, T; Taguchi, Y; Morimatsu, F; Sato, K; Nakamura, Y; Higashi, A; Kido, Y; Nakabo, Y; Ohtsuki, K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem 2006, 53, 6531–6536. [Google Scholar]
- Kamara, MT; Zhu, K; Amadou, I; Tarawalie, F; Zhou, H. Functionality, in vitro digestibility and physicochemical properties of two varieties of defatted foxtail millet protein concentrates. Int. J. Mol. Sci 2009, 10, 5224–5238. [Google Scholar]
- Postlethwaite, AE; Seyer, JM; Kang, AH. Chemotactic attraction of human fibroblasts to type I, II and III collagens and collagen-derived peptides. Proc. Natl. Acad. Sci. USA 1978, 75, 871–875. [Google Scholar]
- Wu, J; Fujioka, M; Sugimoto, K. Assessment of effectiveness of oral administration of collagen peptide on bone metabolism in growing and mature rats. J. Bone Miner. Metab 2004, 22, 547–553. [Google Scholar]
- Minaguchi, J; Koyama, Y; Meguri, N; Hosaka, Y; Ueda, H; Kusubata, M; Hirota, A; Irie, S; Mafune, N; Takehana, K. Effects of ingestion of collagen peptide on collagen fibrils and glycosaminoglycans in Achilles tendon. J. Nutr. Sci. Vitaminol. (Tokyo) 2005, 51, 169–174. [Google Scholar]
- Foh, MBK; Amadou, I; Foh, BM; Kamara, MT; Xia, WS. Functionality and antioxidant properties of tilapia (Oreochromisniloticus) as influenced by the degree of hydrolysis. Int. J. Mol. Sci 2010, 11, 1851–1869. [Google Scholar]
- Alasalvar, C; Shahidi, F; Quantick, P. Food and health applications of marine neutraceuticals. In Seafoods–Quality Technology and Nutraceutical Applications; Alasalvar, C, Taylor, T, Eds.; Springer-Verlag: Berlin, Germany, 2002; pp. 175–204. [Google Scholar]
- Amadou, I; Yong-Hui, S; Sun, J; Guo-Wei, L. Fermented soybean products: some methods, antioxidants compound extraction and their scavenging activity. Asian J. Biochem 2009, 4, 68–76. [Google Scholar]
- Kim, SK; Kim, YT; Byun, HG; Nam, KS; Joo, DS; Shahidi, F. Isolation and Characterization of antioxidative peptides from gelatin hydrolysate of allaskapollack skin. J. Agric. Food Chem 2001, 49, 1984–1989. [Google Scholar]
- Jun, SY; Park, PJ; Jung, WK; Kim, SK. Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limandaaspera) frame protein. Eur. Food Res. Technol 2004, 219, 20–26. [Google Scholar]
- Waszkowiak, K. Antioxidative activity of rosemary extract using connective tissue proteins as carriers. Int. J. Food Sci. Technol 2008, 43, 1437–1442. [Google Scholar]
- Li, B; Chen, F; Wang, X; Ji, B; Wu, Y. Isolation and identification of anti-oxidative peptides from porcine collagen hydrolysates by consecutive chromatography and electrospray ionization—mass spectrometry. Food Chem 2007, 102, 1135–1143. [Google Scholar]
- Lloyd, DJ; Robertson, ME. Structure of collagen fibres and the point of attack by proteolyticenzymes. Nature 1934, 133, 102–103. [Google Scholar]
- Hua, L; Low, TY; Sze, SK. Microwave-assisted specific chemical digestion for rapid protein identification. Proteomics 2006, 6, 586–591. [Google Scholar]
- Zhong, HY. Microwave-assisted acid hydrolysis of proteins combined with liquid chromatography MALDI MS/MS for protein identification. J. Am. Soc. Mass Spectrom 2005, 16, 471–481. [Google Scholar]
- Lin, SS. Microwave-assisted enzyme-catalyzed reactions in various solvent systems. J. Am. Soc. Mass Spectrom 2005, 16, 581–588. [Google Scholar]
- Juan, HF. A new application of microwave technology to proteomics. Proteomics 2005, 5, 840–842. [Google Scholar]
- Vesper, HW. Assessment of microwave-assisted enzymatic digestion by measuring glycated hemoglobin all by massspectrometry. J. Mass Spectrom 2005, 19, 2865–2870. [Google Scholar]
- Stadtman, ER; Oliver, CN. Metal-catalyzed oxidation of protein. Minireview. J. Biol. Chem 1991, 266, 2005–2008. [Google Scholar]
- Stadtman, ER; Berlett, BS. Reactive oxygen-mediated protein oxidation in agingand disease. Chem. Resour. Toxicol 1997, 10, 485–494. [Google Scholar]
- Zhong, HY. Protein sequencing by mass analysis of polypeptide ladders after controlled protein hydrolysis. Nat. Biotechnol 2004, 22, 10–15. [Google Scholar]
- Kim, SK; Kim, YT; Byun, HG; Park, PJ; Ito, H. Purification and characterization of antioxidative peptides from bovineskin. J. Biochem. Mol. Biol 2001, 34, 219–224. [Google Scholar]
- Wu, HC; Chen, HM; Shiau, CY. Free amino acids and peptides as related to antioxidant properties inprotein hydrolysates of mackerel (Scomberaustriasicus). Food Res. Int 2003, 36, 949–957. [Google Scholar]
- Chen, HM; Muramoto, K; Yamauchi, F. Structural analysis of antioxidative peptides from soybean. J. Agric. Food Chem 1995, 43, 574–578. [Google Scholar]
- Rajapakse, N; Mendis, E; Jung, WK; Je, JY; Kim, SK. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int 2005, 38, 175–182. [Google Scholar]
- Mendis, E; Rajapakse, N; Byun, HG; Kim, SK. Investigation of jumbo squid (Dosidicusgigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci 2005, 77, 2166–2178. [Google Scholar]
- Suetsuna, K; Ukeda, H; Ochi, H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J. Nutri. Biochem 2000, 11, 128–131. [Google Scholar]
- Chen, HM; Muramoto, K; Yamauchi, F; Fujimoto, K; Nokihara, K. Anti-oxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybeanprotein. J. Agric. Food Chem 1998, 46, 49–53. [Google Scholar]
- Shimada, K; Fujikawa, K; Yahara, K; Nakamura, T. Antioxidative properties of xanthan on the antioxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem 1992, 40, 945–948. [Google Scholar]
- Re, R; Pellegrini, N; Proteggente, A; Pannala, A; Yang, M; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free Radic. Biol. Med 1999, 26, 1231–1237. [Google Scholar]

| Hydrochloric Acid | Sulfuric Acid | Phosphoric Acid | Citric Acid | |
|---|---|---|---|---|
| DH (%) | 5.22 ± 0.18 | 4.80 ± 0.15 | 6.17 ± 0.08 | 4.16 ± 0.21 |
| ABTS+ (%) | 6.5 ± 0.23 | 4.8 ± 1.02 | 28.0 ± 0.11 | 21.2 ± 0.12 |
| DPPH·(%) | 9.2 ± 0.17 | 12.1 ± 0.09 | 9.9 ± 0.09 | 10.1 ± 0.10 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, Y.-J.; Le, G.-W.; Wang, J.-Y.; Li, Y.-X.; Shi, Y.-H.; Sun, J. Antioxidative Peptides Derived from Enzyme Hydrolysis of Bone Collagen after Microwave Assisted Acid Pre-Treatment and Nitrogen Protection. Int. J. Mol. Sci. 2010, 11, 4297-4308. https://doi.org/10.3390/ijms11114297
Lin Y-J, Le G-W, Wang J-Y, Li Y-X, Shi Y-H, Sun J. Antioxidative Peptides Derived from Enzyme Hydrolysis of Bone Collagen after Microwave Assisted Acid Pre-Treatment and Nitrogen Protection. International Journal of Molecular Sciences. 2010; 11(11):4297-4308. https://doi.org/10.3390/ijms11114297
Chicago/Turabian StyleLin, Yun-Jian, Guo-Wei Le, Jie-Yun Wang, Ya-Xin Li, Yong-Hui Shi, and Jin Sun. 2010. "Antioxidative Peptides Derived from Enzyme Hydrolysis of Bone Collagen after Microwave Assisted Acid Pre-Treatment and Nitrogen Protection" International Journal of Molecular Sciences 11, no. 11: 4297-4308. https://doi.org/10.3390/ijms11114297




