The Evaluation of the Possibilities of Using PLGA Co-Polymer and Its Composites with Carbon Fibers or Hydroxyapatite in the Bone Tissue Regeneration Process – in Vitro and in Vivo Examinations
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Examination on Human Cells
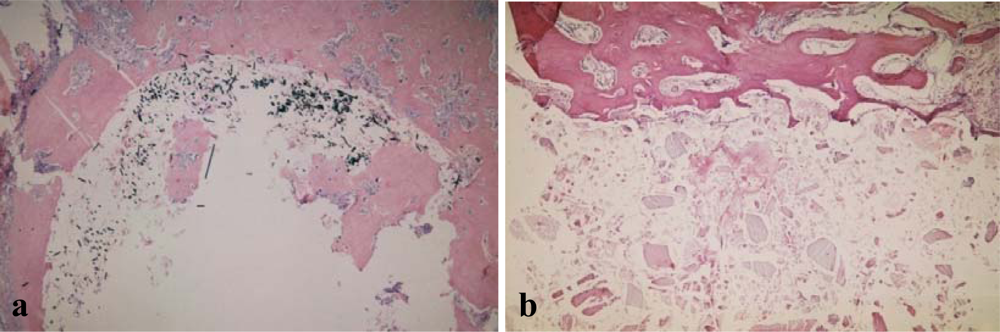
2.2. In Vivo Examinations on Animals
3. Experimental Section
3.1. General
3.2. Samples
3.3. Cell Culture
3.4. LDH (Lactate Dehydrogenase) Release Assay
3.5. In Vivo Experiment
3.6. Histopathological and Immunohistochemical Examination
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Willi, P; Chandra, PS. Nanoceramic matrices: biomedical applications. Am. J. Biochem. Biotechnol 2006, 2, 41–48. [Google Scholar]
- Boccaccini, AR; Blaker, JJ. Bioactive composite materials for tissue engineering scaffolds. Expert Rev. Med. Devic 2005, 2, 303–317. [Google Scholar]
- Wagner, M; Kiapur, N; Wiedmann-Al-Ahmad, M; Hübner, U; Al-Ahmad, A; Shön, R; Schmelzeisen, R; Mülhaupt, R; Gellrich, NC. Comparative in vitro study of the cell proliferation of ovine and human osteoblast-like cells on conventionally and rapid prototyping produced scaffolds tailored for application as potential bone replacement material. J. Biomed. Mater. Res. A 2007, 83, 1154–1164. [Google Scholar]
- Nagata, F; Miyajima, T; Teraoka, K; Yokogawa, Y. Preparation of porous poly(lactic acid)/hydroxyapatite microspheres intended for injectable bone substitutes. Key Eng Mater 2005. [Google Scholar]
- Sosnowski, S; Woźniak, P; Lewandowska-Szumieł, M. Polyester scaffolds with bimodal pore size distribution for tissue engineering. Macromol. Biosci 2006, 6, 425–434. [Google Scholar]
- Kumar, MNVR; Bakowsky, U; Lehr, CM. Preparation and characterization of cationic PLGA nanospheres as DNA carriers. Biomaterials 2004, 25, 1771–1777. [Google Scholar]
- Shi, X; Wang, Y; Ren, L; Gong, Y; Wang, DA. Enhancing alendronate release from a novel PLGA/hydroxyapatite microspheric system for bone repairing applications. Pharm. Res 2009, 26, 422–430. [Google Scholar]
- Balch, OK; Collier, MA; DeBeault, LE; Johnson, LL. Bioabsorbable suture anchor (copolymer 85/15 D,L lactide/glycolide) implanted in bone: correlation of physical/mechanical properties, magnetic resonance imaging, and histological response. Arthroscopy 1999, 15, 691–708. [Google Scholar]
- Tiainen, J; Soini, Y; Tormala, P; Waris, T; Ashammakhi, N. Self-reinforced polylactide/polyglycolide 80/20 screws take more than 1(1/2) years to resorb in rabbit cranial bone. J. Biomed. Mater. Res 2004, 70B, 49–55. [Google Scholar]
- Böstman, O; Pihlajamäki, H. Clinical biocompatibility of biodegradable orthopeadic implants for internal fixation: a review. Biomaterials 2000, 21, 2615–2621. [Google Scholar]
- Jahno, VD; Ribeiro, GB; Dos Santos, LA; Ligabue, R; Einloft, S; Ferreira, MR; Bombonato-Prado, KF. Chemical synthesis and in vitro biocompatibility tests of poly (L-lactic acid). J. Biomed. Mater. Res. A 2007, 83, 209–215. [Google Scholar]
- Bilir, A; Aybar, B; Tanrikulu, SH; Issever, H; Tuna, S. Biocompatibility of different barrier membranes in cultures of human CRL 11372 osteoblast-like cells: an immunohistochemical study. Clin. Oral Implants Res 2007, 18, 46–52. [Google Scholar]
- Di Toro, R; Betti, V; Spampinato, S. Biocompatibility and integrin-mediated adhesion of human osteoblasts to poly(DL-lactide-co-glycolide) copolymers. Eur. J. Pharm. Sci 2004, 21, 161–169. [Google Scholar]
- Chłopek, J; Morawska-Chochół, A; Bajor, G; Adwent, M; Cieślik-Bielecka, A; Cieślik, M; Sabat, D. The influence of carbon fibres on the resorption time and mechanical properties of the lactide-glycolide co-polymer. J. Biomater. Sci. Polym. Ed 2007, 18, 1355–1368. [Google Scholar]
- Pamula, E; Bacakova, L; Filova, E; Buczynska, J; Dobrzynski, P; Noskova, L; Grausova, L. The influence of pore size on colonization of poly(L-lactide-glycolide) scaffolds with human osteoblast-like MG 63 cells in vitro. J. Mater. Sci. Mater. Med 2008, 19, 425–435. [Google Scholar]
- Kokubo, T; Kim, HM; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar]
- Pétas, A; Vuopio-Varkila, J; Siitonen, A; Välimaa, T; Talja, M; Taari, K. Bacterial adherence to self-reinforced polyglycolic acid and self-reinforced polylactic acid 96 urological spiral stents in vitro. Biomaterials 1998, 19, 677–681. [Google Scholar]
- Price, RL; Ellison, K; Haberstroh, KM; Webster, TJ. Nanometr surface roughness increases select osteoblast adhesion on carbon nanofiber compacts. J. Biomed. Mater. Res. A 2004, 70, 129–138. [Google Scholar]
- Graziano, A; d’Aquino, R; Cusella-De Angelis, MG; De Francesco, F; Giordano, A; Laino, G; Piattelli, A; Traini, T; De Rosa, A; Papaccio, G. Scaffold’s surface geometry significantly affects human stem cell bone tissue engineering. J. Cell. Physiol 2008, 214, 166–172. [Google Scholar]
- Wei, G; Ma, PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar]
- Lu, HH; Tang, A; Oh, SC; Spalazzi, JP; Dionisio, K. Compositional effects on the formation of a calcium phosphate layer and the response of osteoblast-like cells on polymer-bioactive glass composites. Biomaterials 2005, 26, 6323–6334. [Google Scholar]
- Athanasiou, KA; Niederauer, GG; Agrawal, CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar]
- Dobrzyński, P; Kasperczyk, J; Janeczek, H. Synthesis of biodegradable copolymers with the use of low toxic zirconium compounds. Copolymerization of glycolide with L-lactide initiated by Zr(Acac)4. Macromolecules 2001, 34, 5090–5099. [Google Scholar]
- Dobrzyński, P; Bero, M; Kasperczyk, J. Synthesis and properties of biodegradable copolymers (PGLA, PGCA, PLCA) obtained in presence of new low toxic initiators containing zircon. Eng Biomater 2001. [Google Scholar]
- Buczyńska, J; Pamuła, E; Błażewicz, S; Bacakova, L; Parizek, M; Chlupac, J; Mikołajczyk, T; Boguń, M; Dobrzyński, P. Fibrous scaffolds for bone tissue engineering: static and dynamic In vitro studiem with MG 63 cells. Eng Biomater 2007. [Google Scholar]
- Shi, X; Wang, Y; Ren, L; Gong, Y; Wang, DA. Enhancing alendronate release from a novel PLGA/hydroxyapatite microspheric system for bone repairing applications. Pharm. Res 2009, 26, 422–430. [Google Scholar]
- Verheggen, R; Merten, HA. Correction of skull defects rusing hydroxyapatite cement (HAC) - evidence derived from animal experiments and clinical experience. Acta Neurochir 2001, 143, 919–926. [Google Scholar]
- PN-EN ISO 10993-5: Biological evaluation of medical devices. In vitro cytotoxicity studies, March 2001.
- Cytotoxicity detection kit (LDH) Instruction manual 5th Version; Roche Applied Science: Germany, 2004.
- Vagaska, B; Bacakova, L; Pamuła, E; Lisa, V; Dobrzyński, P. Adhesion and growth of human osteoblast-like cells on aliphatic polyesters with different chemical compositions, surface roughness and modification with hydroxyapatite. Eng Biomater 2006. [Google Scholar]
- Bostman, O; Hirvensalo, E; Makinen, J; Rokkanen, P. Foreign-body reactions to fracture fixation implants of biodegradable synthetic polymers. J. Bone Joint Surg. Br 1990, 72, 592–598. [Google Scholar]
- Haberko, K; Bućko, M; Haberko, M; Mozgawa, W; Pyda, A; Zarębski, J. Natural hydroxyapatite - preparation, properties. Eng Biomater 2003. [Google Scholar]
| CT [%] – LDH test | ||||||
|---|---|---|---|---|---|---|
| Time | 24 h | 48 h | 72 h | p24–48 | p48–72 | |
| 1 | PLGA | 3.4±2.7 | 4.8±4.1 | 1.1±0.9 | NS | 0.1 |
| 2 | PLGA+CF | 2.5±0.6 | 8.4±2.9 | 0.7±0.7 | <0.01 | <0.01 |
| 3 | PLGA+HA | 10.4±1.6 | 7.0±2.4 | 0.6±1.0 | <0.05 | <0.05 |
| p1–2 | <0.01 | NS | NS | |||
| p1–3 | <0.01 | NS | NS | |||
| Osteonectin / osteopontin activity | |||||
|---|---|---|---|---|---|
| Period | 1st week | 2nd week | 3th week | 6th week | |
| 1 | PLGA | (++) / (++) | (+) / (+) | (+) / (+) | (+) / (+) |
| 2 | PLGA+CF | (+−) / (+−) | (+−) / (+−) | (+−) / (+−) | (+−) / (+−) |
| 3 | PLGA+HA | (++) / (++) | (++) / (++) | (+) / (+) | (+) / (+) |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cieślik, M.; Mertas, A.; Morawska-Chochół, A.; Sabat, D.; Orlicki, R.; Owczarek, A.; Król, W.; Cieślik, T. The Evaluation of the Possibilities of Using PLGA Co-Polymer and Its Composites with Carbon Fibers or Hydroxyapatite in the Bone Tissue Regeneration Process – in Vitro and in Vivo Examinations. Int. J. Mol. Sci. 2009, 10, 3224-3234. https://doi.org/10.3390/ijms10073224
Cieślik M, Mertas A, Morawska-Chochół A, Sabat D, Orlicki R, Owczarek A, Król W, Cieślik T. The Evaluation of the Possibilities of Using PLGA Co-Polymer and Its Composites with Carbon Fibers or Hydroxyapatite in the Bone Tissue Regeneration Process – in Vitro and in Vivo Examinations. International Journal of Molecular Sciences. 2009; 10(7):3224-3234. https://doi.org/10.3390/ijms10073224
Chicago/Turabian StyleCieślik, Magdalena, Anna Mertas, Anna Morawska-Chochół, Daniel Sabat, Rajmund Orlicki, Aleksander Owczarek, Wojciech Król, and Tadeusz Cieślik. 2009. "The Evaluation of the Possibilities of Using PLGA Co-Polymer and Its Composites with Carbon Fibers or Hydroxyapatite in the Bone Tissue Regeneration Process – in Vitro and in Vivo Examinations" International Journal of Molecular Sciences 10, no. 7: 3224-3234. https://doi.org/10.3390/ijms10073224





