Tryptophanase-Catalyzed L-Tryptophan Synthesis from D-Serine in the Presence of Diammonium Hydrogen Phosphate
Abstract
:1. Introduction
2. Results and Discussion
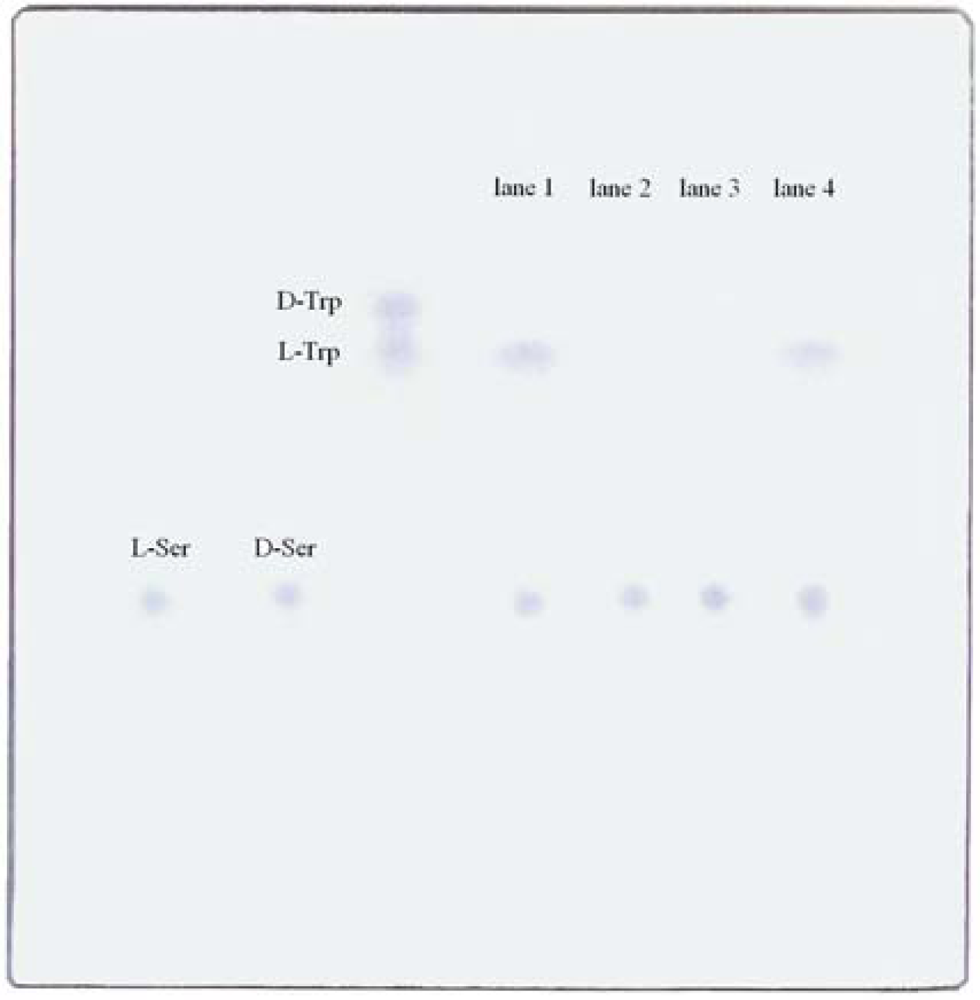
2.1. Reactant product developed on cellulose thin layer chromatography
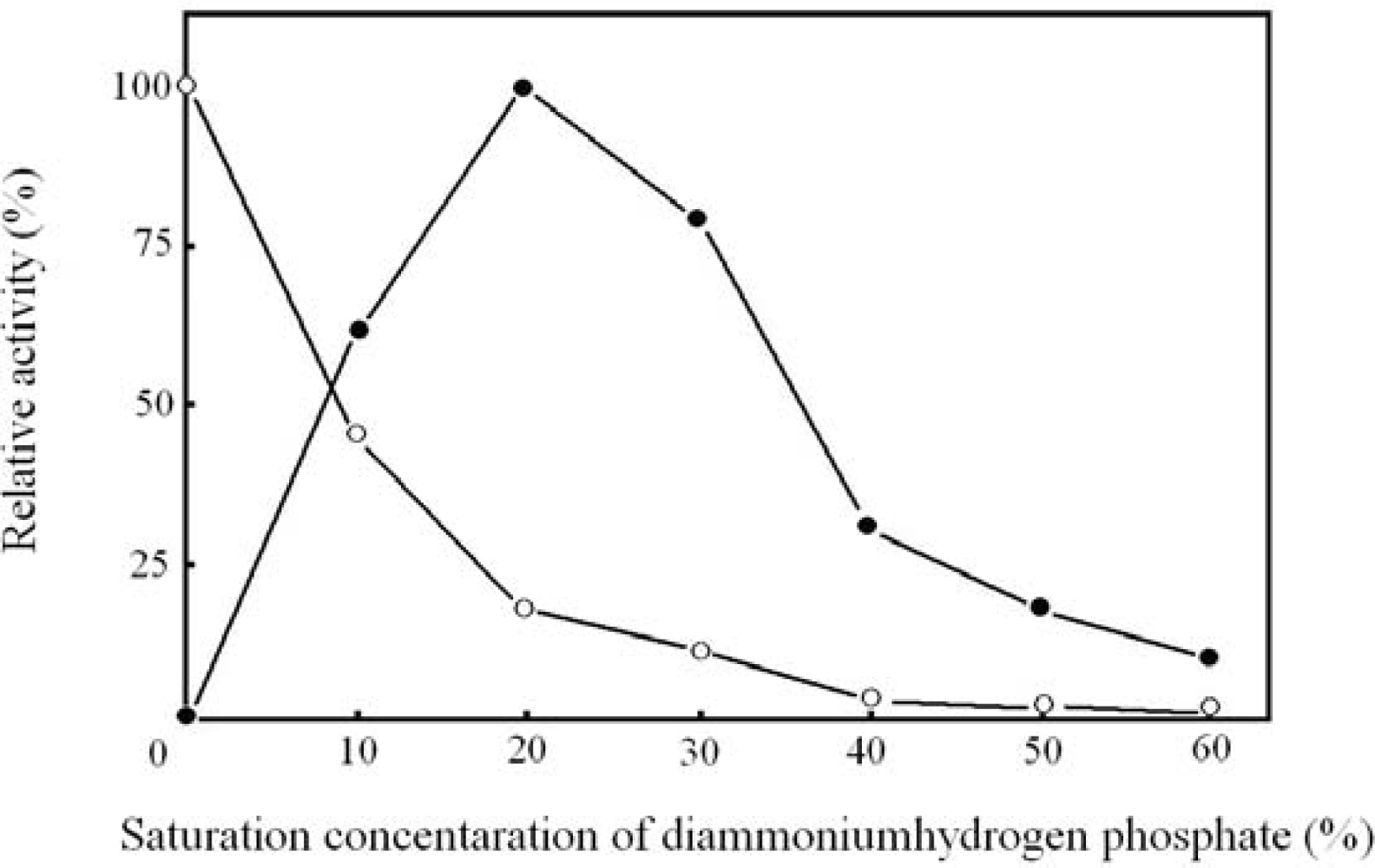
2.2. Optimal concentration of diammoniumhydrogen phosphate
2.3. Effects of other salts on tryptophan synthesis
2.4. Analyses of reaction product with resolution column by UV monitor
2.5. Determination of optical isomeric form by circular dichroism
2.6. Diammoniumhydrogen phosphate efficacy on tryptophan synthesis
2.7. Speculation of l-tryptophan synthesis pathway from d-serine
3. Experimental Section
3.1. Saturated solutions of diammonium phosphate and other salts
3.2. Reaction mixture and reagents
3.3. Thin layer chromatographic analyses
3.4. Tryptophan analyses by use of high pressure liquid chromatography (HPLC)
4. Conclusions
Acknowledgments
References and Notes
- Lahav, M. Question 4: Basic questions about the origin of life: On chirobiogenesis. Orig. Life Evol. Biosph 2007, 37, 371–377. [Google Scholar]
- Bada, JL; Miller, SL. Racemization and the origin of optically active organic compounds in living organisms. Biosystems 1987, 20, 21–26. [Google Scholar]
- Pizzarello, S; Weber, AL. Prebiotic amino acids as asymmetric catalysts. Science 2004, 303, 1151. [Google Scholar]
- Bonner, WA. Chirality and life. Orig. Life Evol. Biosph 1995, 25, 175–190. [Google Scholar]
- Shimada, A; Nakamura, I. Degradation of d-tryptophan by tryptophanase under high salt concentration. Viva Orig 1992, 20, 147–162. [Google Scholar]
- Shimada, A; Shishido, H; Nakamura, I. Tryptophanase-catalysed degradation of d-tryptophan in highly concentrated diammonium hydrogen phosphate solution. Amino Acids 1996, 11, 83–89. [Google Scholar]
- Shimada, A; Kogure, H; Shishido, H; Nakamura, I. Reaction pathway of tryptophanase degrading d-tryptophan. Amino Acids 1997, 12, 379–383. [Google Scholar]
- Shimada, A; Shishido, H; Kogure, H; Nakamura, I; Fuji, N; Akaboshi, M. Tryptophanase-catalyzed d-tryptophan degradation reaction and its significance for chiral homogeneity. In The Role of Radiation in the Origin and Evolution of Life; Akaboshi, M, Ed.; Kyoto University Press: Kyoto, Japan, 2000; pp. 243–257. [Google Scholar]
- Mateus, DMR; Alves, SS; Fonseca, MMR. Kinetics of l-tryptophan production from indole and l-serine catalyzed by whole cells with tryptophanase activity. J. Biosci. Bioeng 2004, 5, 289–293. [Google Scholar]
- Morino, Y; Snell, EE. A kinetic study of the reaction mechanism of tryptophanase-catalyzed reaction. J. Biol. Chem 1967, 242, 2793–2799. [Google Scholar]
- Shimada, A; Yuasa, S. Optical resolution of dl-tryptophan by cellulose. Viva Origino 1979, 8, 23–24. [Google Scholar]
- Yuasa, S; Shimada, A. Separation of dl-amino acids by cellulose thin layer chromatography. Sci. Rep. Osaka Univ 1982, 31, 13–22. [Google Scholar]
- Yuasa, S; Itoh, M; Shimada, A. Resolution of amino acids by native cellulose column. J. Chromatogr. Sci 1984, 22, 287–292. [Google Scholar]
- Shimada, A; Fujii, N; Saito, T. Tryptophanase activity on D-tryptophan. In Progress in Biological Chirality; Paliyi, G, Zucchi, C, Caglioti, L, Eds.; Elsevier: Oxford, UK, 2004; pp. 321–327. [Google Scholar]
- Shimada, A. Role of ammonium phosphates in tryptophanase activity toward d-tryptophan. In d-Amino Acids: A New Frontier in Amino Acid and Protein Research - Practical Methods and Protocols; Konno, R, Brückner, H, D'Aniello, A, Fischer, G, Fujii, N, Homma, H, Eds.; Nova Science Publishers: New York, NY, USA, 2007; pp. 591–607. [Google Scholar]
- Shimada, A. Activity on d-tryptophan attributable to slight conformational change of tryptophanase in highly concentrated ammonium phosphate solution. In Enzymes Involved in the Metabolism of d-Amino Acids: Practical Methods and Protocols; Nova Science Publishers: New York, NY, USA; , in press.
- Okazaki, S; Suzuki, A; Mizushima, T; Komeda, H; Asano, Y; Yamane, T; Okazaki, S; Suzuki, A. Structures of d-amino-acid amidase complexed with l-phenylalanine and with l-phenylalanine amide: insight into the d-stereospecificity of d-amino acid amidase from Ochrobactrum anthropi SV3. Acta Crystallogr. D Biol. Crystallogr 2008, 64, 331–334. [Google Scholar]
- Rentsch, KM. The importance of stereselective determination of drugs in the clinical laboratory. J. Biochem. Biophys. Methods 2002, 54, 1–9. [Google Scholar]
- Ujimaru, T; Kakimoto, T; Chibata, I. l-tryptophan production by Achromobacter liquidum. Appl. Environ. Microbiol 1983, 46, 1–5. [Google Scholar]
- Newton, WA; Morino, Y; Snell, EE. Properties of crystalline tryptophanase. J. Biol. Chem 1965, 240, 1211–1218. [Google Scholar]
- Newton, WA; Snell, EE. Catalytic properties of tryptophanase, a multifunctiomal pyridoxal phosphate enzyme. Proc. Natl. Acad. Sci. USA 1964, 51, 382–389. [Google Scholar]
- Griffiths, SK; DeMoss, RD. Physiological comparison of l-serine dehydratase and tryptophanase from Baccilus alvei. J. Bacteriol 1970, 101, 810–820. [Google Scholar]
- Yuasa, S; Shimada, A; Kameyama, K; Yasui, M; Adzuma, K. Cellulose thin layer and column chromatography for resolution of dl-tryptophan. J. Chromatogr. Sci 1980, 18, 311–314. [Google Scholar]
- Yuasa, S; Itoh, M; Shimada, A. Cellulose column chromatography of dl-tryptophan. Sci. Rep. Osaka Univ 1982, 31, 23–29. [Google Scholar]
- Yuasa, S; Fukuhara, T; Isoyama, M; Shimada, A. Resolution of dl-amino acids on a native cellulose column and a plausible mechanism for their resolution. Biomed. Chromatogr 1997, 11, 276–279. [Google Scholar]


| Salts | tryptophan synthesis |
|---|---|
| NH4Cl | – |
| NH4NO3 | – |
| NH4H2PO4 | – |
| (NH4)2HPO4 | + |
| (NH4)2CO3 | – |
| K2HPO4 | – |
| Na2HPO4 | – |
| NaCl | – |
| Seawater | – |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shimada, A.; Ozaki, H.; Saito, T.; Noriko, F. Tryptophanase-Catalyzed L-Tryptophan Synthesis from D-Serine in the Presence of Diammonium Hydrogen Phosphate. Int. J. Mol. Sci. 2009, 10, 2578-2590. https://doi.org/10.3390/ijms10062578
Shimada A, Ozaki H, Saito T, Noriko F. Tryptophanase-Catalyzed L-Tryptophan Synthesis from D-Serine in the Presence of Diammonium Hydrogen Phosphate. International Journal of Molecular Sciences. 2009; 10(6):2578-2590. https://doi.org/10.3390/ijms10062578
Chicago/Turabian StyleShimada, Akihiko, Haruka Ozaki, Takeshi Saito, and Fujii Noriko. 2009. "Tryptophanase-Catalyzed L-Tryptophan Synthesis from D-Serine in the Presence of Diammonium Hydrogen Phosphate" International Journal of Molecular Sciences 10, no. 6: 2578-2590. https://doi.org/10.3390/ijms10062578




