Polyphenols from Cocoa and Vascular Health—A Critical Review
Abstract
:1. Introduction
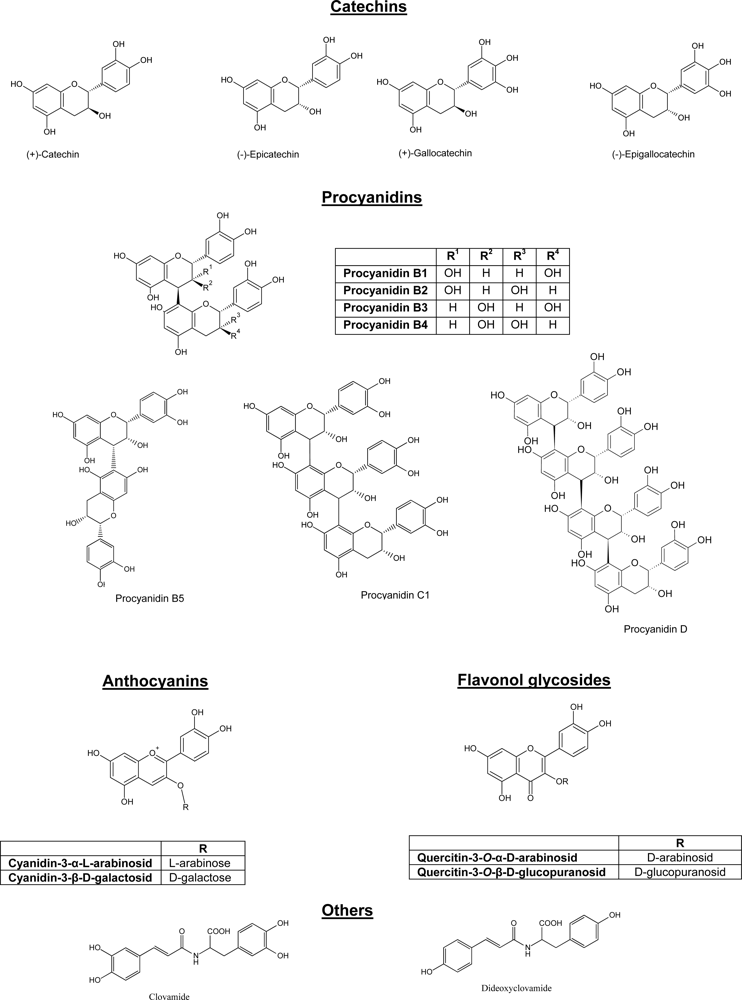
2. Bioavailability of Polyphenols in Cocoa
3. Putative Health Benefits of Polyphenols from Cocoa and Chocolate
3.1. Studies in Vitro and in Cultured Cells
3.2. Studies in Laboratory Animals
3.3. Studies in Humans
4. Conclusions
References and Notes
- Visioli, F; Bernaert, H; Corti, R; Ferri, C; Heptinstall, S; Molinari, E; Poli, A; Serafini, M; Smit, HJ; Vinson, JA; Violi, F; Paoletti, R. Chocolate, lifestyle, and health. Crit. Rev. Food Sci. Nutr 2009, 49, 299–312. [Google Scholar]
- Galleano, M; Oteiza, PI; Fraga, CG. Cocoa, chocolate and cardiovascular disease. J Cardiovasc Pharmacol, 2009; DOI: 10.1097/FJC.0b013e3181b76787. [Google Scholar]
- Corti, R; Flammer, AJ; Hollenberg, NK; Luscher, TF. Cocoa and cardiovascular health. Circulation 2009, 119, 1433–1441. [Google Scholar]
- Baba, S; Osakabe, N; Natsume, M; Yasuda, A; Takizawa, T; Nakamura, T; Terao, J. Cocoa powder enhances the level of antioxidative activity in rat plasma. Br. J. Nutr 2000, 84, 673–680. [Google Scholar]
- Andres-Lacueva, C; Monagas, M; Khan, N; Izquierdo-Pulido, M; Urpi-Sarda, M; Permanyer, J; Lamuela-Raventos, RM. Flavanol and flavonol contents of cocoa powder products: Influence of the manufacturing process. J. Agric. Food Chem 2008, 56, 3111–3117. [Google Scholar]
- Grassi, D; Desideri, G; Necozione, S; Lippi, C; Casale, R; Properzi, G; Blumberg, JB; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr 2008, 138, 1671–1676. [Google Scholar]
- Hammerstone, JF; Lazarus, SA; Mitchell, AE; Rucker, R; Schmitz, HH. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem 1999, 47, 490–496. [Google Scholar]
- Ramiro-Puig, E; Castell, M. Cocoa: Antioxidant and immunomodulator. Br. J. Nutr 2009, 101, 931–940. [Google Scholar]
- Serafini, M; Bugianesi, R; Maiani, G; Valtuena, S; de Santis, S; Crozier, A. Plasma antioxidants from chocolate. Nature 2003, 424, 1013. [Google Scholar]
- Rein, D; Lotito, S; Holt, RR; Keen, CL; Schmitz, HH; Fraga, CG. Epicatechin in human plasma: In vivo determination and effect of chocolate consumption on plasma oxidation status. J. Nutr 2000, 130, 2109S–2114S. [Google Scholar]
- Donovan, JL; Crespy, V; Oliveira, M; Cooper, KA; Gibson, BB; Williamson, G. (+)-Catechin is more bioavailable than (−)-catechin: Relevance to the bioavailability of catechin from cocoa. Free Radic. Res 2006, 40, 1029–1034. [Google Scholar]
- Roura, E; Andres-Lacueva, C; Estruch, R; Mata-Bilbao, ML; Izquierdo-Pulido, M; Waterhouse, AL; Lamuela-Raventos, RM. Milk does not affect the bioavailability of cocoa powder flavonoid in healthy human. Ann. Nutr. Metab 2007, 51, 493–498. [Google Scholar]
- Schramm, DD; Karim, M; Schrader, HR; Holt, RR; Kirkpatrick, NJ; Polagruto, JA; Ensunsa, JL; Schmitz, HH; Keen, CL. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci 2003, 73, 857–869. [Google Scholar]
- Crozier, A; Jaganath, IB; Clifford, MN. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep 2009, 26, 1001–1043. [Google Scholar]
- Steinberg, FM; Bearden, MM; Keen, CL. Cocoa and chocolate flavonoids: Implications for cardiovascular health. J. Am. Diet. Assoc 2003, 103, 215–223. [Google Scholar]
- Richelle, M; Tavazzi, I; Enslen, M; Offord, EA. Plasma kinetics in man of epicatechin from black chocolate. Eur. J. Clin. Nutr 1999, 53, 22–26. [Google Scholar]
- Wang, JF; Schramm, DD; Holt, RR; Ensunsa, JL; Fraga, CG; Schmitz, HH; Keen, CL. A dose-response effect from chocolate consumption on plasma epicatechin and oxidative damage. J. Nutr 2000, 130, 2115S–2119S. [Google Scholar]
- Baba, S; Osakabe, N; Yasuda, A; Natsume, M; Takizawa, T; Nakamura, T; Terao, J. Bioavailability of (−)-epicatechin upon intake of chocolate and cocoa in human volunteers. Free Radic. Res 2000, 33, 635–641. [Google Scholar]
- Wan, Y; Vinson, JA; Etherton, TD; Proch, J; Lazarus, SA; Kris-Etherton, PM. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am. J. Clin. Nutr 2001, 74, 596–602. [Google Scholar]
- Schramm, DD; Wang, JF; Holt, RR; Ensunsa, JL; Gonsalves, JL; Lazarus, SA; Schmitz, HH; German, JB; Keen, CL. Chocolate procyanidins decrease the leukotriene-prostacyclin ratio in humans and human aortic endothelial cells. Am. J. Clin. Nutr 2001, 73, 36–40. [Google Scholar]
- Pearson, DA; Paglieroni, TG; Rein, D; Wun, T; Schramm, DD; Wang, JF; Holt, RR; Gosselin, R; Schmitz, HH; Keen, CL. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb. Res 2002, 106, 191–197. [Google Scholar]
- Holt, RR; Lazarus, SA; Sullards, MC; Zhu, QY; Schramm, DD; Hammerstone, JF; Fraga, CG; Schmitz, HH; Keen, CL. Procyanidin dimer B2 [epicatechin-(4beta-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr 2002, 76, 798–804. [Google Scholar]
- Steinberg, FM; Holt, RR; Schmitz, HH; Keen, CL. Cocoa procyanidin chain length does not determine ability to protect LDL from oxidation when monomer units are controlled. J. Nutr. Biochem 2002, 13, 645–652. [Google Scholar]
- Holt, RR; Schramm, DD; Keen, CL; Lazarus, SA; Schmitz, HH. Chocolate consumption and platelet function. JAMA 2002, 287, 2212–2213. [Google Scholar]
- Murphy, KJ; Chronopoulos, AK; Singh, I; Francis, MA; Moriarty, H; Pike, MJ; Turner, AH; Mann, NJ; Sinclair, AJ. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin. Nutr 2003, 77, 1466–1473. [Google Scholar]
- Waterhouse, AL; Shirley, JR; Donovan, JL. Antioxidants in chocolate. Lancet 1996, 348, 834. [Google Scholar]
- Lotito, SB; Actis-Goretta, L; Renart, ML; Caligiuri, M; Rein, D; Schmitz, HH; Steinberg, FM; Keen, CL; Fraga, CG. Influence of oligomer chain length on the antioxidant activity of procyanidins. Biochem. Biophys. Res. Commun 2000, 276, 945–951. [Google Scholar]
- Richelle, M; Tavazzi, I; Offord, E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J. Agric. Food Chem 2001, 49, 3438–3442. [Google Scholar]
- Osakabe, N; Yasuda, A; Natsume, M; Takizawa, T; Terao, J; Kondo, K. Catechins and their oligomers linked by C4 → C8 bonds are major cacao polyphenols and protect low-density lipoprotein from oxidation in vitro. Exp. Biol. Med. (Maywood) 2002, 227, 51–56. [Google Scholar]
- Vinson, JA; Proch, J; Zubik, L. Phenol antioxidant quantity and quality in foods: Cocoa, dark chocolate, and milk chocolate. J. Agric. Food Chem 1999, 47, 4821–4824. [Google Scholar]
- Schewe, T; Sadik, C; Klotz, LO; Yoshimoto, T; Kuhn, H; Sies, H. Polyphenols of cocoa: Inhibition of mammalian 15-lipoxygenase. Biol. Chem 2001, 382, 1687–1696. [Google Scholar]
- Schewe, T; Kuhn, H; Sies, H. Flavonoids of cocoa inhibit recombinant human 5-lipoxygenase. J. Nutr 2002, 132, 1825–1829. [Google Scholar]
- Hatano, T; Miyatake, H; Natsume, M; Osakabe, N; Takizawa, T; Ito, H; Yoshida, T. Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects. Phytochemistry 2002, 59, 749–758. [Google Scholar]
- Mao, T; van de Water, J; Keen, CL; Schmitz, HH; Gershwin, ME. Cocoa procyanidins and human cytokine transcription and secretion. J. Nutr 2000, 130, 2093S–2099S. [Google Scholar]
- Mao, TK; Powell, J; van de Water, J; Keen, CL; Schmitz, HH; Gershwin, ME. Effect of cocoa procyanidins on the secretion of interleukin-4 in peripheral blood mononuclear cells. J. Med. Food 2000, 3, 107–114. [Google Scholar]
- Mao, TK; Powell, J; van de Water, J; Keen, CL; Schmitz, HH; Hammerstone, JF; Gershwin, ME. The effect of cocoa procyanidins on the transcription and secretion of interleukin 1 beta in peripheral blood mononuclear cells. Life Sci 2000, 66, 1377–1386. [Google Scholar]
- Mao, TK; van de Water, J; Keen, CL; Schmitz, HH; Gershwin, ME. Modulation of TNF-alpha secretion in peripheral blood mononuclear cells by cocoa flavanols and procyanidins. Dev. Immunol 2002, 9, 135–141. [Google Scholar]
- Mao, TK; van de Water, J; Keen, CL; Schmitz, HH; Gershwin, ME. Effect of cocoa flavanols and their related oligomers on the secretion of interleukin-5 in peripheral blood mononuclear cells. J. Med. Food 2002, 5, 17–22. [Google Scholar]
- Guo, Q; Rimbach, G; Packer, L. Nitric oxide formation in macrophages detected by spin trapping with iron-dithiocarbamate complex: Effect of purified flavonoids and plant extracts. Methods Enzymol 2001, 335, 273–282. [Google Scholar]
- Saliou, C; Valacchi, G; Rimbach, G. Assessing bioflavonoids as regulators of NF-kappa B activity and inflammatory gene expression in mammalian cells. Methods Enzymol 2001, 335, 380–387. [Google Scholar]
- Ramiro, E; Franch, A; Castellote, C; Andres-Lacueva, C; Izquierdo-Pulido, M; Castell, M. Effect of Theobroma cacao flavonoids on immune activation of a lymphoid cell line. Br. J. Nutr 2005, 93, 859–866. [Google Scholar] [Green Version]
- Ramiro, E; Franch, A; Castellote, C; Perez-Cano, F; Permanyer, J; Izquierdo-Pulido, M; Castell, M. Flavonoids from Theobroma cacao down-regulate inflammatory mediators. J. Agric. Food Chem 2005, 53, 8506–8511. [Google Scholar]
- Park, YC; Rimbach, G; Saliou, C; Valacchi, G; Packer, L. Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNF-alpha secretion, and NF-kappaB-dependent gene expression in RAW 264.7 macrophages. FEBS Lett 2000, 465, 93–97. [Google Scholar]
- Schnorr, O; Brossette, T; Momma, TY; Kleinbongard, P; Keen, CL; Schroeter, H; Sies, H. Cocoa flavanols lower vascular arginase activity in human endothelial cells in vitro and in erythrocytes in vivo. Arch. Biochem. Biophys 2008, 476, 211–215. [Google Scholar]
- Karim, M; McCormick, K; Kappagoda, CT. Effects of cocoa extracts on endothelium-dependent relaxation. J. Nutr 2000, 130, 2105S–2108S. [Google Scholar]
- Lee, KW; Kang, NJ; Oak, MH; Hwang, MK; Kim, JH; Schini-Kerth, VB; Lee, HJ. Cocoa procyanidins inhibit expression and activation of MMP-2 in vascular smooth muscle cells by direct inhibition of MEK and MT1-MMP activities. Cardiovasc. Res 2008, 79, 34–41. [Google Scholar]
- Actis-Goretta, L; Ottaviani, JI; Keen, CL; Fraga, CG. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett 2003, 555, 597–600. [Google Scholar]
- Kurosawa, T; Itoh, F; Nozaki, A; Nakano, Y; Katsuda, S; Osakabe, N; Tsubone, H; Kondo, K; Itakura, H. Suppressive effect of cocoa powder on atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. J. Atheroscler. Thromb 2005, 12, 20–28. [Google Scholar]
- Vinson, JA; Proch, J; Bose, P; Muchler, S; Taffera, P; Shuta, D; Samman, N; Agbor, GA. Chocolate is a powerful ex vivo and in vivo antioxidant, an antiatherosclerotic agent in an animal model, and a significant contributor to antioxidants in the European and American Diets. J. Agric. Food Chem 2006, 54, 8071–8076. [Google Scholar]
- Yasuda, A; Natsume, M; Sasaki, K; Baba, S; Nakamura, Y; Kanegae, M; Nagaoka, S. Cacao procyanidins reduce plasma cholesterol and increase fecal steroid excretion in rats fed a high-cholesterol diet. Biofactors 2008, 33, 211–223. [Google Scholar]
- Jalil, AM; Ismail, A; Pei, CP; Hamid, M; Kamaruddin, SH. Effects of cocoa extract on glucometabolism, oxidative stress, and antioxidant enzymes in obese-diabetic (ob-db) rats. J. Agric. Food Chem 2008, 56, 7877–7884. [Google Scholar]
- Orozco, TJ; Wang, JF; Keen, CL. Chronic consumption of a flavanol- and procyanindin-rich diet is associated with reduced levels of 8-hydroxy-2′-deoxyguanosine in rat testes. J. Nutr. Biochem 2003, 14, 104–110. [Google Scholar]
- Kenny, TP; Keen, CL; Schmitz, HH; Gershwin, ME. Immune effects of cocoa procyanidin oligomers on peripheral blood mononuclear cells. Exp. Biol. Med. (Maywood) 2007, 232, 293–300. [Google Scholar]
- Rein, D; Paglieroni, TG; Pearson, DA; Wun, T; Schmitz, HH; Gosselin, R; Keen, CL. Cocoa and wine polyphenols modulate platelet activation and function. J. Nutr 2000, 130, 2120S–2126S. [Google Scholar]
- Mackenzie, GG; Carrasquedo, F; Delfino, JM; Keen, CL; Fraga, CG; Oteiza, PI. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-kappaB activation at multiple steps in Jurkat T cells. FASEB J 2004, 18, 167–169. [Google Scholar]
- Zhu, QY; Holt, RR; Lazarus, SA; Orozco, TJ; Keen, CL. Inhibitory effects of cocoa flavanols and procyanidin oligomers on free radical-induced erythrocyte hemolysis. Exp. Biol. Med. (Maywood) 2002, 227, 321–329. [Google Scholar]
- Kondo, K; Hirano, R; Matsumoto, A; Igarashi, O; Itakura, H. Inhibition of LDL oxidation by cocoa. Lancet 1996, 348, 1514. [Google Scholar]
- Osakabe, N; Baba, S; Yasuda, A; Iwamoto, T; Kamiyama, M; Takizawa, T; Itakura, H; Kondo, K. Daily cocoa intake reduces the susceptibility of low-density lipoprotein to oxidation as demonstrated in healthy human volunteers. Free Radic. Res 2001, 34, 93–99. [Google Scholar]
- Baba, S; Osakabe, N; Kato, Y; Natsume, M; Yasuda, A; Kido, T; Fukuda, K; Muto, Y; Kondo, K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr 2007, 85, 709–717. [Google Scholar]
- Mursu, J; Voutilainen, S; Nurmi, T; Rissanen, TH; Virtanen, JK; Kaikkonen, J; Nyyssonen, K; Salonen, JT. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Radic. Biol. Med 2004, 37, 1351–1359. [Google Scholar]
- Rein, D; Paglieroni, TG; Wun, T; Pearson, DA; Schmitz, HH; Gosselin, R; Keen, CL. Cocoa inhibits platelet activation and function. Am. J. Clin. Nutr 2000, 72, 30–35. [Google Scholar]
- Wiswedel, I; Hirsch, D; Kropf, S; Gruening, M; Pfister, E; Schewe, T; Sies, H. Flavanol-rich cocoa drink lowers plasma F(2)-isoprostane concentrations in humans. Free Radic. Biol. Med 2004, 37, 411–421. [Google Scholar]
- Fisher, ND; Hughes, M; Gerhard-Herman, M; Hollenberg, NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J. Hypertens 2003, 21, 2281–2286. [Google Scholar]
- Heiss, C; Dejam, A; Kleinbongard, P; Schewe, T; Sies, H; Kelm, M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 2003, 290, 1030–1031. [Google Scholar]
- Heiss, C; Kleinbongard, P; Dejam, A; Perre, S; Schroeter, H; Sies, H; Kelm, M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J. Am. Coll. Cardiol 2005, 46, 1276–1283. [Google Scholar]
- Schroeter, H; Heiss, C; Balzer, J; Kleinbongard, P; Keen, CL; Hollenberg, NK; Sies, H; Kwik-Uribe, C; Schmitz, HH; Kelm, M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar]
- Vlachopoulos, C; Aznaouridis, K; Alexopoulos, N; Economou, E; Andreadou, I; Stefanadis, C. Effect of dark chocolate on arterial function in healthy individuals. Am J Hypertens 2005, 18, 785–791. [Google Scholar]
- Faridi, Z; Njike, VY; Dutta, S; Ali, A; Katz, DL. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nutr 2008, 88, 58–63. [Google Scholar]
- Fraga, CG. Cocoa, diabetes, and hypertension: Should we eat more chocolate? Am. J. Clin. Nutr 2005, 81, 541–542. [Google Scholar]
- Grassi, D; Lippi, C; Necozione, S; Desideri, G; Ferri, C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr 2005, 81, 611–614. [Google Scholar]
- Grassi, D; Necozione, S; Lippi, C; Croce, G; Valeri, L; Pasqualetti, P; Desideri, G; Blumberg, JB; Ferri, C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005, 46, 398–405. [Google Scholar]
- Taubert, D; Berkels, R; Roesen, R; Klaus, W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA 2003, 290, 1029–1030. [Google Scholar]
- Taubert, D; Roesen, R; Lehmann, C; Jung, N; Schomig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA 2007, 298, 49–60. [Google Scholar]
- Balzer, J; Rassaf, T; Heiss, C; Kleinbongard, P; Lauer, T; Merx, M; Heussen, N; Gross, HB; Keen, CL; Schroeter, H; Kelm, M. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J. Am. Coll. Cardiol 2008, 51, 2141–2149. [Google Scholar]
- Farouque, HM; Leung, M; Hope, SA; Baldi, M; Schechter, C; Cameron, JD; Meredith, IT. Acute and chronic effects of flavanol-rich cocoa on vascular function in subjects with coronary artery disease: A randomized double-blind placebo-controlled study. Clin. Sci. (Lond) 2006, 111, 71–80. [Google Scholar]
- Mehrinfar, R; Frishman, WH. Flavanol-rich cocoa: A cardioprotective nutraceutical. Cardiol. Rev 2008, 16, 109–115. [Google Scholar]
- Flammer, AJ; Hermann, F; Sudano, I; Spieker, L; Hermann, M; Cooper, KA; Serafini, M; Luscher, TF; Ruschitzka, F; Noll, G; Corti, R. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation 2007, 116, 2376–2382. [Google Scholar]
- Turner, R; Baron, T; Wolffram, S; Minihane, AM; Cassidy, A; Rimbach, G; Weinberg, PD. Effect of circulating forms of soy isoflavones on the oxidation of low density lipoprotein. Free Radic. Res 2004, 38, 209–216. [Google Scholar]
- Rimbach, G; Weinberg, PD; de Pascual-Teresa, S; Alonso, MG; Ewins, BA; Turner, R; Minihane, AM; Botting, N; Fairley, B; Matsugo, S; Uchida, Y; Cassidy, A. Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochim. Biophys. Acta 2004, 1670, 229–237. [Google Scholar]
- Halliwell, B. Plasma antioxidants: Health benefits of eating chocolate. Nature 2003, 426, 787. [Google Scholar]
- Mathur, S; Devaraj, S; Grundy, SM; Jialal, I. Cocoa products decrease low density lipoprotein oxidative susceptibility but do not affect biomarkers of inflammation in humans. J. Nutr 2002, 132, 3663–3667. [Google Scholar]
- Innes, AJ; Kennedy, G; McLaren, M; Bancroft, AJ; Belch, JJ. Dark chocolate inhibits platelet aggregation in healthy volunteers. Platelets 2003, 14, 325–327. [Google Scholar]
- Hermann, F; Spieker, LE; Ruschitzka, F; Sudano, I; Hermann, M; Binggeli, C; Luscher, TF; Riesen, W; Noll, G; Corti, R. Dark chocolate improves endothelial and platelet function. Heart 2006, 92, 119–120. [Google Scholar]
- Fisher, ND; Hollenberg, NK. Aging and vascular responses to flavanol-rich cocoa. J. Hypertens 2006, 24, 1575–1580. [Google Scholar]
- Wang-Polagruto, JF; Villablanca, AC; Polagruto, JA; Lee, L; Holt, RR; Schrader, HR; Ensunsa, JL; Steinberg, FM; Schmitz, HH; Keen, CL. Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women. J Cardiovasc Pharmacol 2006, 47(Suppl. 2), S177–S186. [Google Scholar]
- Heiss, C; Finis, D; Kleinbongard, P; Hoffmann, A; Rassaf, T; Kelm, M; Sies, H. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J. Cardiovasc. Pharmacol 2007, 49, 74–80. [Google Scholar]
- Mohan, S; Campbell, NR. Salt and high blood pressure. Clin. Sci. (Lond) 2009, 117, 1–11. [Google Scholar]
- Davison, K; Coates, AM; Buckley, JD; Howe, PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int. J. Obes. (Lond) 2008, 32, 1289–1296. [Google Scholar]
- Ferri, C; Grassi, G. Mediterranean diet, cocoa and cardiovascular disease: A sweeter life, a longer life, or both? J. Hypertens 2003, 21, 2231–2234. [Google Scholar]
- Grassi, D; Desideri, G; Croce, G; Tiberti, S; Aggio, A; Ferri, C. Flavonoids, vascular function and cardiovascular protection. Curr. Pharm. Des 2009, 15, 1072–1084. [Google Scholar]
- Joshipura, KJ; Hu, FB; Manson, JE; Stampfer, MJ; Rimm, EB; Speizer, FE; Colditz, G; Ascherio, A; Rosner, B; Spiegelman, D; Willett, WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann. Intern. Med 2001, 134, 1106–1114. [Google Scholar]
- Hertog, MG; Feskens, EJ; Hollman, PC; Katan, MB; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen elderly study. Lancet 1993, 342, 1007–1011. [Google Scholar]
- Erdman, JW, Jr; Balentine, D; Arab, L; Beecher, G; Dwyer, JT; Folts, J; Harnly, J; Hollman, P; Keen, CL; Mazza, G; Messina, M; Scalbert, A; Vita, J; Williamson, G; Burrowes, J. Flavonoids and heart health. In Proceedings of the ILSI North America Flavonoids Workshop, Washington, DC, USA, May 31–June 1, 2005. J. Nutr 2007, 137, 718S–737S. [Google Scholar]
- Buijsse, B; Feskens, EJ; Kok, FJ; Kromhout, D. Cocoa intake, blood pressure, and cardiovascular mortality: The Zutphen elderly study. Arch. Intern. Med 2006, 166, 411–417. [Google Scholar]
- Mink, PJ; Scrafford, CG; Barraj, LM; Harnack, L; Hong, CP; Nettleton, JA; Jacobs, DR, Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr 2007, 85, 895–909. [Google Scholar]
- Hooper, L; Kroon, PA; Rimm, EB; Cohn, JS; Harvey, I; Le Cornu, KA; Ryder, JJ; Hall, WL; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr 2008, 88, 38–50. [Google Scholar]
- Janszky, I; Mukamal, KJ; Ljung, R; Ahnve, S; Ahlbom, A; Hallqvist, J. Chocolate consumption and mortality following a first acute myocardial infarction: The Stockholm heart epidemiology program. J. Intern. Med 2009, 266, 248–257. [Google Scholar]
- Cooper, KA; Donovan, JL; Waterhouse, AL; Williamson, G. Cocoa and health: A decade of research. Br. J. Nutr 2008, 99, 1–11. [Google Scholar]
- Mullen, W; Borges, G; Donovan, JL; Edwards, CA; Serafini, M; Lean, ME; Crozier, A. Milk decreases urinary excretion but not plasma pharmacokinetics of cocoa flavan-3-ol metabolites in humans. Am. J. Clin. Nutr 2009, 89, 1784–1791. [Google Scholar]
- Hollenberg, NK; Fisher, ND. Is it the dark in dark chocolate? Circulation 2007, 116, 2360–2362. [Google Scholar]
| No. Subjects | Age range (mean) | BMI (kg/m2) | Dietary source of polyphenols | Polyphenol content | Plasma concentration | Reference |
|---|---|---|---|---|---|---|
| 8 | 25–55 (40 ± 15) (± SD) | 23.94 ± 2.35 (± SD) | Dark chocolate: 40 g 80 g | Epicatechin: 82 mg 164 mg | Epicatechin: 0.383 μmol/L (t = 2 h) 0.7 μmol/L (t = 2.57 h) | [16] |
| 20 | 20–56 | 23.8 ± 0.79 (± SEM) | Semi-sweet chocolate baking bits: 27 g 53 g 80 g | Total procyanidins (epicatechin): 186 (46) mg 365 (90) mg 551 (136) mg | Epicatechin (t = 2 h): 0.133 μmol/L 0.258 μmol/L 0.355 μmol/L | [17] |
| 13 | 26–49 | 23.2 ± 1.2 (± SEM) | 105 g semi-sweet chocolate baking bits (of which 80 g procyanidin-rich chocolate) | 557 mg total procyanidins (137 mg epicatechin) | 0.257 μmol/L epicatechin (t = 2 h) | [10] |
| 5 | 30–33 (31 ± 1) (± SD) | 22.5 ± 1.3 (± SD) | 96 g chocolate 66 g cocoa | Total polyphenols (epicatechin): 2.74 g (760 μmoL) 2.73 g (760 μmoL) | Total epicatechin (t = 2 h): 4.77 μmol/L 4.92 μmol/L | [18] |
| 23 | 21–62 | – | 22 g cocoa powder + 16 g dark chocolate | 466 mg total procyanidins (111 mg monomers) | 0.036 nmol/L epicatechin (t = 2 h) | [19] |
| 11 | 20–55 (39 ± 5) (± SD) | 24 ± 3 (± SD) | 37 g high- vs. low-procyanidin chocolate | Total procyanidins: 4 mg/g vs. 0.9 mg/g | 0.212 μmol/L vs. 0.011 μmol/L epicatechin (t = 2 h) | [20] |
| 16 | 22–49 | – | 300 mL cocoa beverage (18.75 g flavanol-rich cocoa powder) | 897 mg total epicatechin & procyanidins | 1.043 μmol/L epicatechin (t=2 h) | [21] |
| 5 | 23–34 | – | Cocoa beverage (0.375 g cocoa/kg bw) | Per g cocoa: 12.2 mg monomers, 9.7 mg dimers, 28.2 mg procyanidins | 0.041 μmol/L dimer B2, 5.92 μmol/L epicatechin, 0.16 μmol/L catechin (t = 2 h) | [22] |
| 6 | 23–39 | 23.1 ± 0.7 (± SEM) | 400 mL flavanol-rich cocoa beverage (37.5 g cocoa); 2 days | Per g cocoa: 12.2 mg monomers, 9.7 mg dimers, 20.2 mg procyanidins | 0.08 μmol/L dimer B2, 4.11 μmol/L epicatechin, 0.4 μmol/L catechin (t = 2 h) | [23] |
| 18 | – | – | 25 g semi-sweet chocolate chips | 220 mg flavanols & procyanidins | 0.427 μmol/L epicatechin (t = 2 h) | [24] |
| 32 | 31–49 (40 ± 9) (± SD) | 26 ± 4 (± SD) | Cocoa flavanol & procyanidin supplementation for 28 days | 234 mg/d flavanols & procyanidins | 0.116 μmol/L epicatechin 0.091 nmol/L catechin (t = 28 d) | [25] |
| Cell type | Treatment | Outcomes | Reference |
|---|---|---|---|
| Human LDL | Cocoa powder extract (5 μmol/L GAE°) vs. pure catechin (5 μmol/L) | LDL oxidation ↓ | [26] |
| Liposomes & human LDL | Cocoa catechin monomers & procyanidin fractions (0.1–10.0 μg/mL) | LDL oxidation ↓ | [27] |
| LDL | 220 mL cocoa drink (cocoa concentration: 1.5, 2.0, 2.5, 3.0, 3.5%) | LDL oxidation ↓ dose-dependent | [28] |
| LDL | Catechin, epicatechin, procyanidin B2, procyanidin C1, cinnamtannin A2 (0.125, 0.25, 0.5, 1.0, 2.0 μg/mL) | LDL oxidizability ↓ | [29] |
| Human LDL & VLDL | Dark chocolate, cocoa, milk chocolate, hot cocoa mixes (126, 224, 52.2, 8.2 μmol/g total phenols) | Lag time of LDL & VLDL oxidation ↑ | [30] |
| LDL & VLDL | Dark chocolate & cocoa powder (containing fat or defatted) vs. cocoa butter | LDL & VLDL oxidizability ↓ | [49] |
| Rat liver microsomes | Cacao liquor | NADPH-dependent lipid peroxidation ↓ Linoleic acid autoxidation ↓ | [33] |
| Recombinant human 5-LOX | Cocoa epicatechin & procyanidins (10 μmol/L) | 5-LOX activity ↓ Proinflammatory mediators (LTB4, LTC4, LTD4) ↓ | [32] |
| Isolated rabbit 15-LOX-1 Recombinant human platelet 12-LOX | Cocoa procyanidins (monomers through decamers; 2.9 mg/mL) Epicatechin & procyanidin decamer | 15-LOX-1 activity ↓ dose-dependent 12-LOX activity ↓ dose-dependent | [31] |
| Human PBMC * | Cocoa procyanidins (monomers through decamers; 25 μg/mL) | IL-1β secretion ↑ IL-2 expression ↓ IL-4 expression & secretion ↓ | [34] |
| Human PBMC * | Cocoa procyanidins (monomers through decamers; 25 μg/mL) | IL-1β transcription & secretion ↑ (pentamers-decamers) IL-1β transcription & secretion ↓ (monomers-tetramers) | [36] |
| Human PBMC * | Cocoa procyanidins (monomers through decamers; 25 μg/mL) | Secretory IL-4 ↑ | [35] |
| Human PBMC * | Cocoa procyanidins (monomers through decamers; 25 μg/mL) | TNF-α secretion ↑ | [37] |
| Human PBMC * | Cocoa procyanidins (monomers through decamers; 25 μg/mL) | IL-5 secretion ↑ (monomers-trimers) IL-5 secretion ↓ (hexamers-decamers) | [38] |
| Human PBMC° | Short- (monomers-pentamers) & long-chain (hexamers-decamers) flavanol fractions (20 μg/mL) | Inflammatory mediators (IL-1β, IL-6, IL-10, TNF-α) ↑ | [53] |
| Whole blood | Purified trimeric & pentameric cocoa procyanidins (3, 10 μmol/L) | PAC-1 binding & P-selectin expression ↑ in unstimulated platelets Epinephrine-induced platelet activation ↓ | [54] |
| Isolated rabbit aortic rings | Procyanidin-rich cocoa extracts | Endothelium-dependent relaxation ↑ NOS activity ↑ | [45] |
| ACE from rabbit lung | Epicatechin, dimeric & hexameric procyanidins (0–500 μmol/L) | ACE activity ↓ molecular weight-dependent | [47] |
| Murine EL4BOU6 lymphocytes | Cocoa extract (5–80 μg/mL total polyphenols) vs. epicatechin (60–120 μg/mL total polyphenols) | IL-2 secretion ↓ IL-4 secretion↑ T lymphocyte activation ↓ | [41] |
| Murine RAW264.7 macrophages # Rat NR8383 macrophages # | Cocoa extract (5–100 μg/mL total polyphenols) vs. epicatechin (60–120 μg/mL total polyphenols) Cocoa extract (10–50 μg/mL total polyphenols) vs. epicatechin (30–60 μg/mL total polyphenols) | inducible NO ↓ Proinflammatory mediators (TNF-α, MCP-1, IL-1α, IL-6) ↓ | [42] |
| Jurkat T cells | Catechin, epicatechin & B-type oligomers (1.7–17.2 μmol/L) | PMA-induced NF-κB activation ↓ IL-2 expression & secretion ↓ | [55] |
| VSMC | Cocoa procyanidins (0–100 μg/mL) & procyanidin B2 (0–100 μmol/L) | MMP-2 expression & activation ↓ VSMC invasion & migration ↓ MT1-MMP & MEk1 activities ↓ | [46] |
| HUVEC | Epicatechin & flavanol metabolites mixture vs. control | Arginase-2 mRNA expression ↓ Arginase activity ↓ dose-dependent | [44] |
|
| Species | No. animals | Experimental duration (wk) | Treatment | Outcomes | Reference |
|---|---|---|---|---|---|
| Rats | 5 | – | Oral administration of 1 g cocoa powder/kg bw (7.8 mg epicatechin) | Lipid peroxides in plasma ↓ Oxidant-induced α-tocopherol consumption ↓ | [4] |
| Rats | 5–6 | – | Gastric intubation with 100 mg cocoa extract | Plasma antioxidant capacity ↑ Erythrocyte hemolysis ↓ | [56] |
| Rats | 48 | 2 | Diet containing by weight 0.5–2.0% flavanol- and procyanidin-rich cocoa (12.2 mg/g epicatechin, 2.8 mg/g catechin, 53.3 mg/g procyanidins) | Oxidative DNA damage in testes ↓ No effect on plasma F2-isoprostane and TBARS | [52] |
| Hypercholesterolemic rabbits | 12 | 24 | Diet containing 10% cocoa powder (0.78 g total polyphenols) | TBARS in plasma↓ No effects on plasma cholesterol, TG & phospholipids LDL oxidation lag time ↑ Area of atherosclerotic lesions in aorta ↓ | [48] |
| Hamsters | 27 | 10 | Brownie (10 g vs. 1 g cocoa powder) | LDL & TG levels ↓ HDL ↑ dose-dependent LDL-oxidizability ↓ | [49] |
| Obese-diabetic rats | 40 | 4 | Cocoa extract (600 mg/kg bw/d) | Blood glucose levels ↓ Plasma free fatty acids ↓ 8-isoprostane levels ↓ Superoxide dismutase activity ↑ No change in catalase activity | [51] |
| Rats | 10 | 4 | 40 g cocoa powder per kg diet (11.0 mg/g epicatechin, 2.8 mg/g catechin, 43.0 mg/g procyanidins), vs. none | Renal arginase activity ↓ | [44] |
| No. Subjects | Age range (mean) | BMI (kg/m2) | Intervention | Polyphenol content | Outcomes | Reference |
|---|---|---|---|---|---|---|
| 12 | 39 ± 4.0 | – | Cocoa | – | LDL oxidation ↓ | [57] |
| 15 | (32.5 ± 6.4) | 21.7 ± 2.1 | 12 g cocoa powder x3/d for 2 weeks, vs. sugar | 2610 mg total polyphenols/d (244 mg epicatechin) | LDL oxidation ↓ No change in plasma lipids or antioxidants Urinary excretion of epicatechin/metabolites ↑ | [58] |
| 23 | 21–62 (36) | – | 22 g cocoa powder + 16 g dark chocolate/d for 4 weeks, vs. average American diet | 466 mg procyanidins/d (111 mg monomers) | LDL oxidation ↓ Serum antioxidant capacity ↑ HDL cholesterol ↑ | [19] |
| 25 | 20–6 (32.4 ± 7.4) | 24.4 ± 3.4 | 37 g dark chocolate & 31 g cocoa powder in a drink/d for 6 weeks, vs. none | 651 mg total procyanidins/d (chocolate = 168 mg/d, cocoa = 483 mg/d) | LDL oxidizability ↓ No effect on inflammation markers, or plasma antioxidant capacity | [81] |
| 45 | 19–49 (26) | 21.5 ± 2.9 /24.1 ± 3.5 | 75 g dark chocolate or high-phenolic dark chocolate for 3 weeks, vs. 75 g white chocolate | Total polyphenols (epicatechin): Dark = 274 (114) mg/d High = 418 (170) mg/d | HDL cholesterol ↑ Lipid peroxidation ↓ No change in plasma antioxidant capacity | [60] |
| 25 | (38 ± 1) | 22.1 ± 0.4 | 26 g/d cocoa powder for 12 weeks | Per 100 g: 377 mg epicatechin, 135 mg catechin, 158 mg procyanidin B2, 96.1 mg procyanidin C1 | LDL oxidation ↓ HDL-cholesterol ↑ | [59] |
| 20 | 20–56 | 23.8 ± 0.79 | Semi-sweet chocolate baking bits (27, 53, 80 g), vs. none | Total procyanidins (epicatechin): 186 (46) mg 365 (90) mg 551 (136) mg | Plasma epicatechin ↑ dose-dependent Antioxidant capacity ↑ TBARS ↓ | [17] |
| 13 | 26–49 | 23.2 ± 1.2 | 105 g (of which 80 g chocolate) semi-sweet baking bits, vs. vanilla milk chips | 557 mg total procyanidins (137 mg epicatechin) | Plasma epicatechin ↑ Total antioxidant capacity ↑ TBARS ↓ | [10] |
| 20 | 20–40 | – | 100 ml high- vs. low-flavanol cocoa drink | 187 mg vs. 14 mg total monomers & oligomeric procyanidins | Plasma F2-isoprostanes ↓ | [62] |
| 12 | 25–35 (32.2 ± 1.0) | 21.9 ± 0.4 | 100 g dark chocolate (with & without 200 mL milk), vs. 200 g milk chocolate | Not stated but FRAP values (147.4 μmol FE/100 g) | Plasma antioxidant capacity & epicatechin ↑, in absence of milk | [9] |
| 30 | 24–49 | – | Cocoa beverage (300 ml, 18.75 g procyanidin-rich cocoa powder), caffeinated beverage, or water | 897 mg total epicatechin & oligomeric procyanidins | Platelet activation & function ↓ | [61] |
| 18 | – | – | 25 g semi-sweet chocolate chips, vs. none | 220 mg flavanols & procyanidins | Plasma epicatechin ↑ Platelet function ↓ | [24] |
| 32 | 40 ± 9 | 26 ± 4 | Cocoa flavanol/procyanidin tablets for 28 d, vs. placebo | 234 mg flavanols & procyanidins/d | Platelet aggregation ↓ Plasma ascorbic acid ↑ No change in oxidation status markers Plasma epicatechin & catechin ↑ | [25] |
| 30 | 20–58 (30.6) | – | Dark (75% cocoa), vs. milk (20% cocoa) or white (no flavonoids) chocolate High polyphenol vs. low | – | Collagen–induced platelet aggregation ↓ | [82] |
| 27 | 18–72 (44 ± 3.4) | 26.9 ± 0.9 | flavanol cocoa drink (4x 230 mL/d for 4 d) | 821 mg/d total flavanols (epicatechin, catechin & related oligomers) | Improved peripheral vasodilation | [63] |
| 20 | 41 ± 14 | 25 ± 4 | 100 mL high polyphenol vs. low flavanol cocoa drink | 176 mg total flavanols (70 mg monomers, 106 mg procyanidins) | NO bioactivity ↑ Arterial FMD ↑ | [64] |
| 10 | – | – | 200 mL high- vs. low-flavanol cocoa beverage | 985 vs. 80.4 mg total flavanols | Erythrocyte arginase activity ↓ | [44] |
| 13 | 55–64 | 21.9–26.2 | 100 g dark chocolate/d for 14 d, vs. 90 g white chocolate in hypertensive subjects | 500 mg/d total polyphenols | Systolic & diastolic blood pressure ↓ | [72] |
| 15 | (33.9 ± 7.6) | 22.6 ± 3.0 | 100 g dark chocolate, vs. 90 g white chocolate for 15 d | 500 mg total polyphenols | Insulin sensitivity ↑ Insulin resistance ↓ Systolic blood pressure ↓ | [70] |
| 28 | – | – | 105 g/d milk chocolate for 14 d, vs. cocoa butter chocolate; hypertensive subjects | 168 mg/d flavanols (39 mg monomers, 126 mg polymers) 2.62 g procyanidins | Diastolic & mean blood pressure ↓ LDL cholesterol ↓ Oxidative stress markers ↓ | [69] |
| 17 | 24–32 (28.9) | <27.0 | 100 g dark chocolate, vs. none | (0.54 g monomers & dimers, 0.76 g trimer-heptamers) | Improved endothelial function Vasodilation of brachial artery No change in blood pressure | [67] |
| 20 | (43.65 ± 7.8) | 25.4 ± 1.7 | 100 g/d dark chocolate for 15 d, vs. 90 g white chocolate in hypertensive subjects | 88 mg/d flavanols (22 mg catechin, 66 mg epicatechin) | Improved insulin sensitivity Systolic & diastolic blood pressure ↓ LDL cholesterol ↓ Improved FMD | [71] |
| 11 | (31 ± 1) | 21.8 ± 0.8 | 100 mL high- vs. low-polyphenol cocoa drink | 176–185 mg flavanols (70–74 mg monomers, 20–22 mg epicatechin, 106–111 mg procyanidins) | Circulating NO & FMD ↑ | [65] |
| 16 | 25–32 | 19–23 | 300 mL high- vs. low-flavanol cocoa drink | 917 mg flavanols (19% epicatechin) | Circulating NO species ↑ FMD response of conduit arteries ↑ Microcirculation ↑ | [66] |
| 20 | – | – | 40 g dark chocolate, vs. white chocolate | Same brand as used for Vlachopoulos et al. (2005) | Improved FMD Platelet function ↓ Plasma total antioxidant status ↑ | [83] |
| 34 | 18–74 (47.9 ± 3.0) | 28.0 ± 1.9/28.4 ± 1.3 | High polyphenol cocoa drink 4x 230 mL/d for 4–6 d, vs. none | Per 100 mL: 9.2 mg epicatechin, 10.7 mg catechin, 69.3 mg flavanol oligomers (821 mg/d) | NO synthesis ↓ FMD ↑ Pulse wave amplitude ↑ | [84] |
| 40 | 61 ± 9 | 27.1 ± 3.9 | 48 g flavanol-rich chocolate bar + 18 g cocoa beverage/d, vs. placebo for 6 weeks in subjects with coronary artery disease | 444 vs. 19.6 mg/d total flavanols (107 vs. 4.7 mg epicatechin) | No acute or chronic changes in FMD, systemic arterial compliance, forearm blood flow, soluble cellular adhesion molecules | [75] |
| 32 | 57.7 ± 2.2/55.4 ± 1.7 | 24.9 ± 1.0/25.3 ± 0.8 | High- vs. low-flavanol cocoa beverage for 6 weeks in hypercholesterolemic subjects | 446 vs. 43 mg total flavanols | FMD ↑ Brachial artery hyperaemic blood flow ↑ VCAM-1 ↓ | [85] |
| 11 | 22–32 (27 ± 1) | 22 ± 1 | 100 mL high-flavanol vs. low-phenolic cocoa drink x3/d for 1 week | Per 100 ml: 59 mg epicatechin, 15 mg catechin, 232 mg flavanol oligomers (918 mg/d procyanidins) | FMD ↑ No change in biomarkers of oxidative stress | [86] |
| 45 | 30–75 (52.8 11.0) ± | 30.1 ± 3.3 | 74 g solid dark chocolate (22 g cocoa powder); 240 ml liquid cocoa (sugar-free vs. sugared) | 821 mg total flavanols; 805.2 & 8.5 mg total flavanols | Improved FMD Systolic & diastolic blood pressure ↓ | [68] |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rimbach, G.; Melchin, M.; Moehring, J.; Wagner, A.E. Polyphenols from Cocoa and Vascular Health—A Critical Review. Int. J. Mol. Sci. 2009, 10, 4290-4309. https://doi.org/10.3390/ijms10104290
Rimbach G, Melchin M, Moehring J, Wagner AE. Polyphenols from Cocoa and Vascular Health—A Critical Review. International Journal of Molecular Sciences. 2009; 10(10):4290-4309. https://doi.org/10.3390/ijms10104290
Chicago/Turabian StyleRimbach, Gerald, Mona Melchin, Jennifer Moehring, and Anika E. Wagner. 2009. "Polyphenols from Cocoa and Vascular Health—A Critical Review" International Journal of Molecular Sciences 10, no. 10: 4290-4309. https://doi.org/10.3390/ijms10104290





