New Imide 5-HT1A Receptor Ligands – Modification of Terminal Fragment Geometry
Abstract
:Introduction
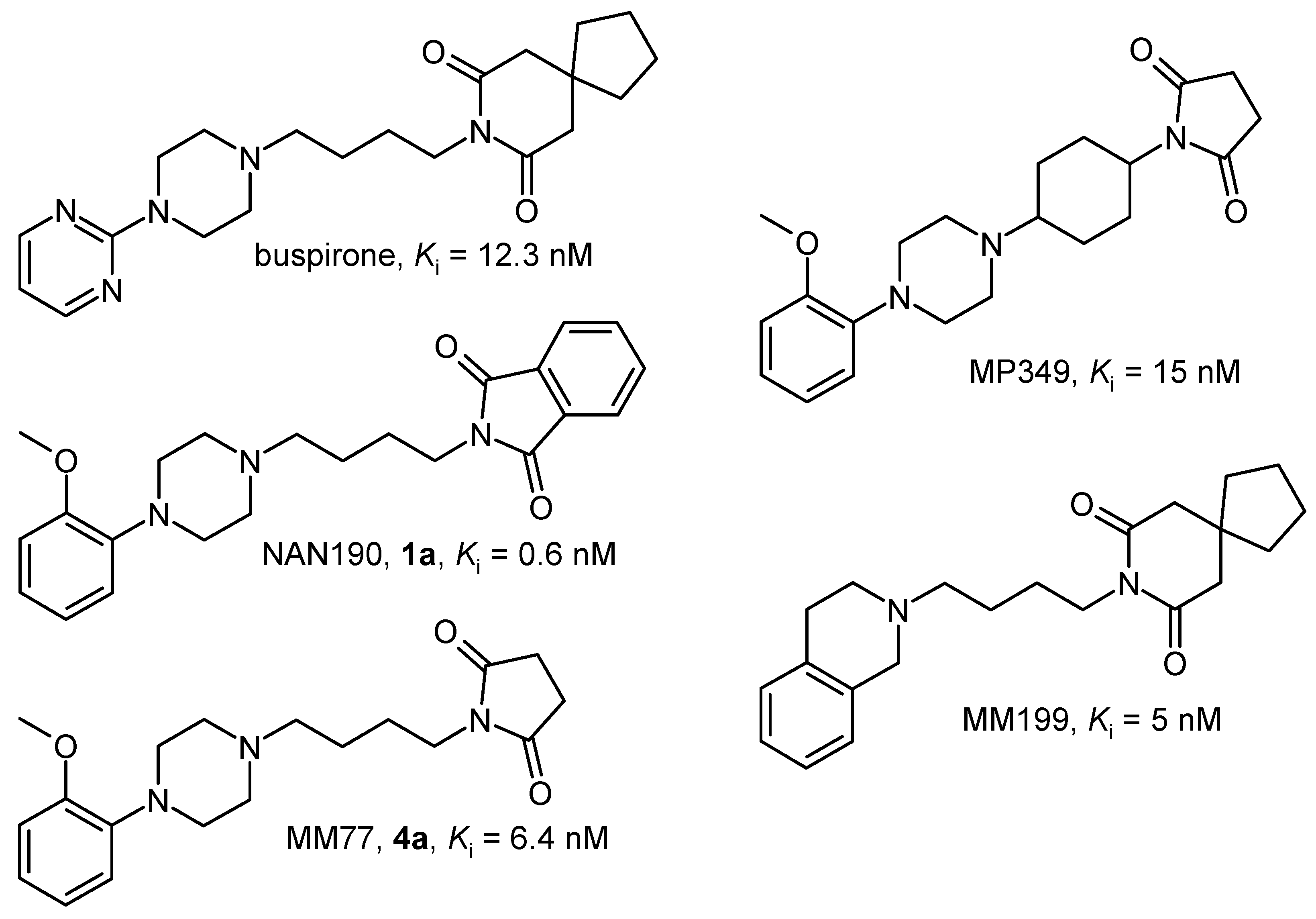
Results and Discussion
| No. | Imide moiety | a: |  | b: |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki ± SEM (nM) | Ki ± SEM (nM) | |||||||||||||
| 5-HT1A | 5-HT2A | 5-HT1A | 5-HT2A | |||||||||||
| 1 |  | 0.55±0.14a,b | 451±65 | 355±28b | 2770±130 | |||||||||
| 2 |  | 4.0±0.2b | 109±22 | 50±11b | 880±12 | |||||||||
| 3 |  | 1.7±0.5 | 185±42 | 157±17 | 1780±25 | |||||||||
| 4 |  | 6.4±0.3c | 1510±95 | 2920±90d | NT | |||||||||
| 5 |  | 6.8±0.5 | 690±40 | 453±15 | 4000±30 | |||||||||
| 6 |  | 7.5±0.7 | 835±45 | 690±20 | 4400±40 | |||||||||
| 7 |  | 46±3 | 86±2 | 620±18 | 1230±34 | |||||||||
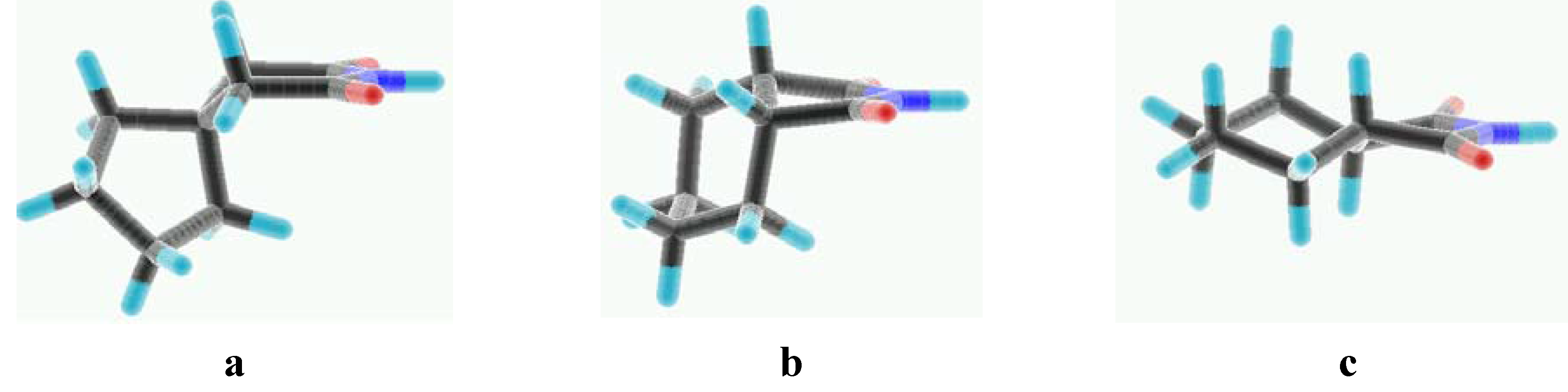
Experimental
General
General method for the preparation of compounds 3a, 3b, 5a, 5b, 6a, 6b, 7a and 7b.
4-{4-[2-(trans-1,2-Cyclohexanedicarboxyimido)]butyl}-1-(2-methoxyphenyl)piperazine (3a).
1-(2-Methoxyphenyl)-4-[4-(3-phenylsuccinimido)butyl]piperazine (5a).
1-(2-Methoxyphenyl)-4-[4-(3-phenylmaleicimido)butyl]piperazine (6a).
1-(2-Methoxyphenyl)-4-[4-(1,8-naphthalimido)butyl)]piperazine (7a).
2-{4-[2-(trans-1,2-Cyclohexanedicarboxyimido)]butyl}-1,2,3,4-tetrahydroisoquinoline (3b).
2-[4-(3-Phenylsuccinimido)butyl]-1,2,3,4-tetrahydroisoquinoline (5b).
2-[4-(3-phenylmaleicimido)butyl]-1,2,3,4-tetrahydroisoquinoline (6b).
2-[4-(1,8-naphthalimido)butyl]-1,2,3,4-tetrahydroisoquinoline (7b).
Radioligand binding studies
References
- Lopez-Rodriguez, M.L.; Ayala, D.; Benhamu, B.; Morcillo, M.J.; Viso, A. Arylpiperazine Derivatives Acting at 5-HT1A Receptors. Curr. Med. Chem. 2002, 9, 443. [Google Scholar]
- Goa, K.L.; Ward, A. Buspirone a preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs 1986, 32, 114. [Google Scholar]
- Glennon, R.A.; Naiman, N.A.; Pierson, M.E.; Titeler, M.; Lyon, R.A.; Weisberg, E. NAN-190: an arylpiperazine analog that antagonizes the stimulus effects of the 5-HT1A agonist 8-hydroxy-2-(di-n- propylamino)tetralin (8-OH- DPAT). Eur. J. Pharmacol. 1988, 154, 339. [Google Scholar]
- Zifa, E.; Fillion, G. 5-Hydroxytryptamine receptors. Pharmacol. Rev. 1992, 44, 401. [Google Scholar]
- Mokrosz, M.J.; Chojnacka-Wójcik, E.; Tatarczyńska, E.; Kłodzińska, A.; Filip, M.; Boksa, J.; Charakchieva-Minol, S.; Mokrosz, J.L. 1-(2-Methoxyphenyl)-4-[(4-succinimido)butyl]piperazine (MM-77): A New, Potent, Postsynaptic Antagonist of 5-HT1A Receptors. Med. Chem. Res. 1994, 4, 161. [Google Scholar]
- Paluchowska, M.H.; Bojarski, A.J.; Charakchieva-Minol, S.; Wesołowska, A. Active Conformation of Some Arylpiperazine Postsynaptic 5-HT1A Receptor Antagonists. Eur. J. Med. Chem. 2002, 37, 273. [Google Scholar]
- Wesołowska, A.; Paluchowska, M.H.; Gołębiowska, K.; Chojnacka-Wójcik, E. Pharmacological Characterization of MP 349, a Novel 5-HT1A Receptor Antagonist with Anxiolytic-Like Activity, in Mice and Rats. J. Pharm. Pharmacol. 2003, 55, 533. [Google Scholar]
- Griebel, G.; Rodgers, R.J.; Perrault, G.; Sanger, D.J. The Effects of Compounds Varying in Selectivity as 5-HT1A Receptor Antagonists in Three Rat Models of Anxiety. Neuropharmacology 2000, 39, 1848. [Google Scholar]
- Mokrosz, J.L.; Dereń-Wesołek, A.; Tatarczyńska, E.; Duszyńska, B.; Bojarski, A.J.; Mokrosz, M.J.; Chojnacka-Wójcik, E. 8-{4-[2-(1,2,3,4-Tetrahydroisoquinolinyl)]-butyl}-9-azaspiro[4.5]-decane-7,9-dione: A New 5-HT1A Receptor Ligand with the Same Activity Profile as Buspirone. J. Med. Chem. 1996, 39, 1125. [Google Scholar]
- Deren-Wesolek, A.; Tatarczynska, E.; Chojnacka-Wojcik, E. The novel buspirone analogue, 8-[4-[2-(1,2,3,4-tetrahydroisoquinolinyl)[butyl]-8-azaspiro [4.5]decane-7,9-dione, with anxiolytic-like and antidepressant-like effects in rats. J. Psychopharmacol. 1998, 12, 380. [Google Scholar]
- Paluchowska, M.H.; Mokrosz, M.J.; Bojarski, A.J.; Wesołowska, A.; Borycz, J.; Charakchieva-Minol, S.; Chojnacka-Wójcik, E. On the bioactive conformation of NAN-190 (1) and MP3022 (2), 5-HT(1A) receptor antagonists. J. Med. Chem. 1999, 42, 4952. [Google Scholar]
- Mokrosz, J.L.; Mokrosz, M.J.; Bojarski, A.J.; Charakchieva-Minol, S. Structure-Activity Relationship Studies of CNS Agents. Part 16: A Lower Limit of a Distance Between Crucial Pharmacophores of 5-HT1A Ligands. Pharmazie 1994, 49, 781. [Google Scholar]
- Mokrosz, M.J.; Bojarski, A.J.; Duszyńska, B.; Tatarczyńska, E.; Kłodzińska, A.; Dereń-Wesołek, A.; Charakchieva-Minol, S.; Chojnacka-Wójcik, E. 1,2,3,4-Tetrahydroisoquinoline derivatives: a new class of 5-HT1A receptor ligands. Bioorg. Med. Chem. 1999, 7, 287. [Google Scholar]
- Hackling, A.; Ghosh, R.; Perachon, S.; Mann, A.; Holtje, H.D.; Wermuth, C.G.; Schwartz, J.C.; Sippl, W.; Sokoloff, P.; Stark, H. N-(omega-(4-(2-methoxyphenyl)piperazin-1-yl)alkyl)carboxamides as dopamine D2 and D3 receptor ligands. J. Med. Chem. 2003, 46, 3883. [Google Scholar]
- Glennon, R.A.; Naiman, N.A.; Pierson, M.E.; Smith, J.D.; Ismaiel, A.M.; Titeler, M.; Lyon, R.A. N-(phthalimidoalkyl) derivatives of serotonergic agents: a common interaction at 5-HT1A serotonin binding sites? J. Med. Chem. 1989, 32, 1921. [Google Scholar]
- Bojarski, A.J.; Cegła, M.T.; Charakchieva-Minol, S.; Mokrosz, M.J.; Maćkowiak, M.; Mokrosz, J.L. Structure-Activity Relationship Studies of CNS Agents. Part 9: 5-HT1A and 5-HT2 Receptor Affinity of Some 2- and 3-Substituted 1,2,3,4-Tetrahydro-β-carbolines. Pharmazie 1993, 48, 289. [Google Scholar]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099. [Google Scholar]
- Sample Availability: Available from the authors.
© 2004 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Bojarski, A.J.; Mokrosz, M.J.; Duszyńska, B.; Kozioł, A.; Bugno, R. New Imide 5-HT1A Receptor Ligands – Modification of Terminal Fragment Geometry. Molecules 2004, 9, 170-177. https://doi.org/10.3390/90300170
Bojarski AJ, Mokrosz MJ, Duszyńska B, Kozioł A, Bugno R. New Imide 5-HT1A Receptor Ligands – Modification of Terminal Fragment Geometry. Molecules. 2004; 9(3):170-177. https://doi.org/10.3390/90300170
Chicago/Turabian StyleBojarski, Andrzej J., Maria J. Mokrosz, Beata Duszyńska, Aneta Kozioł, and Ryszard Bugno. 2004. "New Imide 5-HT1A Receptor Ligands – Modification of Terminal Fragment Geometry" Molecules 9, no. 3: 170-177. https://doi.org/10.3390/90300170




