Biaryl Product Formation from Cross-coupling in Palladiumcatalyzed Borylation of a Boc Protected Aminobromoquinoline Compound
Abstract
:Introduction

Results and Discussion
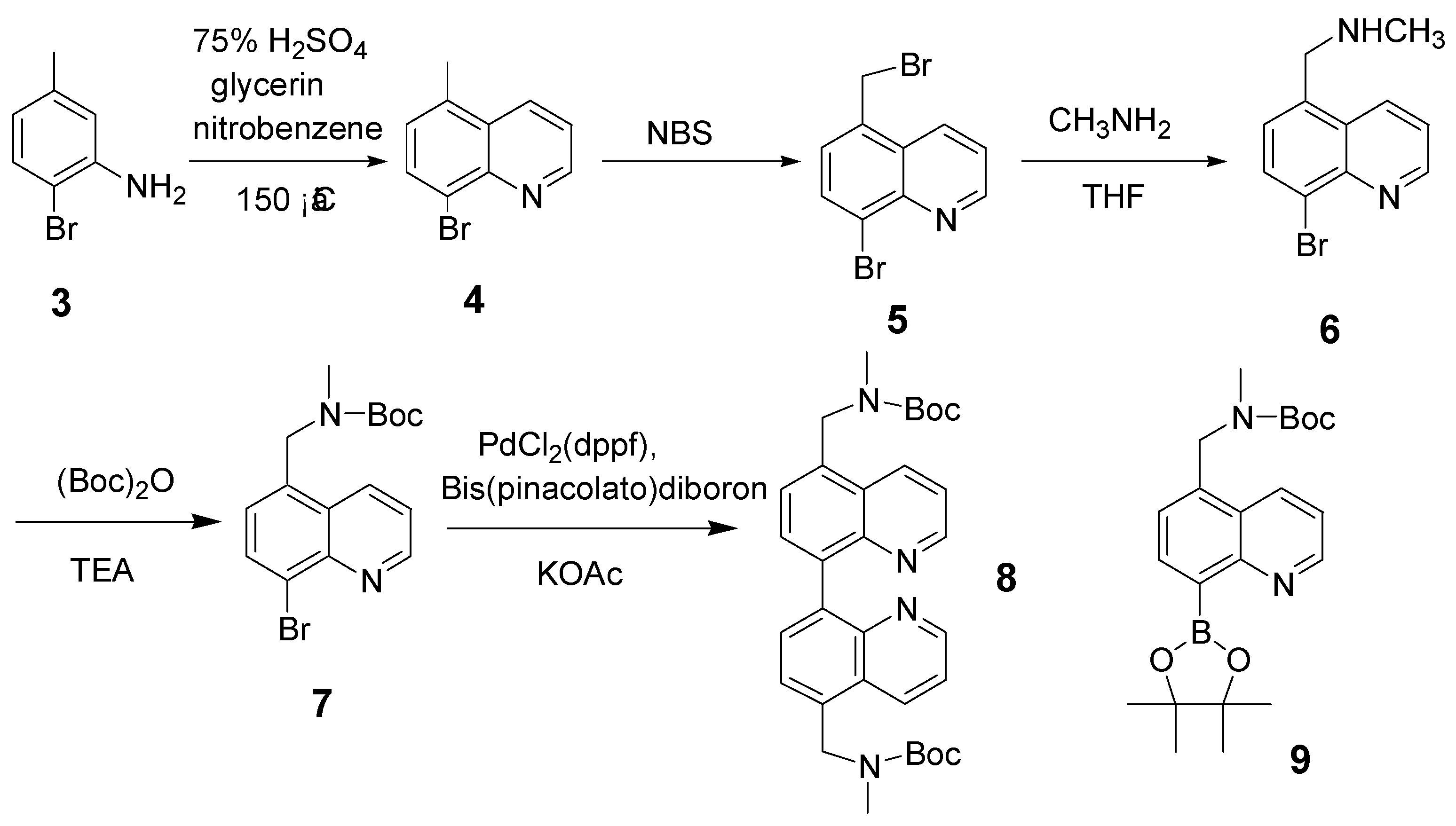
Conclusions
Experimental
General
8-Bromo-5-methylquinoline (4)
8-Bromo-5-bromomethylquinoline (5).
(8-Bromoquinolin-5-yl-methyl)methylamine (6).
Tert-butyl N-(8-bromoquinolin-5-yl-methyl)-N-methylcarbamate (7).
5,5’-Bis[[(tert-butoxycarbonyl)methylamino]methyl]-8,8’-biquinoline (8).
Acknowledgements
References
- Martin, R. F.; Haigh, A.; Monger, C.; Pardee, M.; Whittaker, A. D.; Kelly, D. P.; Allen, B. J. Progress in Neutron Capture Therapy for Cancer; AllenB., J., Moore, D. E., Harrington, B. V., Eds.; Plenum Press: New York, 1992; p. 357. [Google Scholar]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457. [Google Scholar]
- Ishihara, K.; Yamamoto, H. Arylboron Compounds as Acid Catalysts in Organic Synthetic Transformations. Eur. J. Org. Chem. 1999, 527–538. [Google Scholar]
- Latta, R. P.; Springsteen, G.; Wang, B. Development of an Arylboronic Acid-based Solid-Phase Amidation Catalyst. Synthesis 2001, 1611–1613. [Google Scholar]
- Petasis, N. A.; Zavialov, I. A. A New and Practical Synthesis of α-Amino Acids from Alkenyl Boronic Acids. J. Am. Chem. Soc. 1997, 119, 445–446. [Google Scholar]
- Yu, H.; Wang, B. Phenylboronic Acids Facilitated Selective Reduction of Aldehydes by Tributyltin Hydride. Synth. Commun. 2001, 31, 163–169. [Google Scholar]
- Ferrier, R. J. Carbohydrate Boronates. Adv. Cabohydr. Chem. Biochem. 1978, 35, 31. [Google Scholar]
- Yang, W.; Gao, X.; Wang, B. Boronic Acid Compounds as Potential Pharmaceutical Agents. Med. Res. Rev. 2003, 23, 346–368. [Google Scholar]
- Wang, W.; Gao, X.; Wang, B. Boronic Acid-based Sensors for Carbohydrates. Curr. Org. Chem. 2002, 6, 1285–1317. [Google Scholar]
- James, T. D.; Shinkai, S. Artificial Receptors as Chemosensors for Carbohydrates. Top. Curr. Chem. 2002, 218, 159–200. [Google Scholar]
- Shinkai, S.; Takeuchi, M. Molecular Design of Artificial Sugar Sensing Systems. Trend in Anal. Chem. 1996, 15, 188–193. [Google Scholar]
- Lorand, J. P.; Edwards, J. O. Polyol Complexes and Structure of the Benzeneboronate Ion. J. Org. Chem. 1959, 24, 769. [Google Scholar]
- Springsteen, G.; Wang, B. A Detailed Examination of Boronic Acid-Diol Complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar]
- Wang, W.; Gao, S.; Wang, B. Building Fluorescent Sensors by Template Polymerization: The Preparation of a Fluorescent Sensor for D-Fructose. Org. Lett. 1999, 1, 1209–1212. [Google Scholar]
- Liao, Y.; Wang, W.; Wang, B. Building Fluorescent Sensors by Template Polymerization: The Preparation of a Fluorescent Sensor for L-Tryptophan. Bioorg. Chem. 1999, 27, 463–476. [Google Scholar]
- Gao, S.; Wang, W.; Wang, B. Building Fluorescent Sensors for Carbohydrates Using Template-directed Polymerizations. Bioorg. Chem. 2001, 29, 308–320. [Google Scholar]
- Yang, W.; Gao, S.; Gao, X.; Karnati, V. R.; Ni, W.; Wang, B.; Hooks, W. B.; Carson, J.; Weston, B. Diboronic Acids as Fluorescent Probes for Cells Expressing Sialyl Lewis X. Bioorg. Med. Chem. Lett. 2002, 12, 2175–2177. [Google Scholar]
- Yang, W.; Springsteen, G.; Yan, J.; Deeter, S.; Wang, B. A Novel Type of Fluorescent Boronic Acid that Shows Large Fluorescence Intensity Changes upon Binding with a Diol in Aqueous Solution at Physiological pH. Bioorg. Med. Chem. Lett. 2003, 13, 1019–1022. [Google Scholar]
- Karnati, V.; Gao, X.; Gao, S.; Yang, W.; Sabapathy, S.; Ni, W.; Wang, B. A Selective Fluorescent Sensor for Glucose. Bioorg. Med. Chem. Lett. 2002, 12, 3373–3377. [Google Scholar]
- Yang, W.; Fan, H.; Gao, S.; Gao, X.; Ni, W.; Karnati, V.; Hooks, W. B.; Carson, J.; Weston, B.; Wang, B. The First Fluorescent Diboronic Acid Sensor Specific for Hepatocellular Carcinoma Cells Expressing Sialyl Lewis X. Chem. Biol. 2004. manuscript accepted. [Google Scholar]
- Yang, W.; Yan, J.; Fang, H.; Wang, B. The First Fluorescent Sensor for D-Glucarate Based on the Cooperative Action of Boronic Acid and Guanidinium Groups. Chem. Commun. 2003, 792–793. [Google Scholar]
- Gao, X.; Zhang, Y.; Wang, B. New Boronic Acid Fluorescent Reporter Compounds II. A Naphthalene-based Sensor Functional at Physiological pH. Org. Lett. 2003, 5, 4615–4618. [Google Scholar]
- Ni, W.; Fang, H.; Springsteen, G.; Wang, B. Design of Boronic Acid Spectroscopic Reporter Compounds by Taking Advantage of the pKa-Lowering Effect of Diol-binding: Nitrophenol-based Color Reporters for Diols. J. Org. Chem. 2004. manuscript accepted. [Google Scholar]
- Miyaura, N.; Maruoka, K. Synthesis of Organometallic Compounds; Komiya, S., Ed.; Wiley: New York, 1997. [Google Scholar]
- Ishiyama, T.; Murata, M.; Miyaura, N. Palladium(0)-Catalyzed Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters. J. Org. Chem. 1995, 60, 7508–7510. [Google Scholar]
- Murata, M.; Oyama, T.; Watanabe, S.; Masuda, Y. Palladium-catalyzed Borylation of Aryl Halides or Triflates with Dialkoxyborane: A Novel and Facile Synthetic Route to Arylboronates. J. Org. Chem. 2000, 65, 164–168. [Google Scholar]
- Ishiyama, T.; Itoh, Y.; Kitano, T.; Miyaura, N. Synthesis of Arylboronates via the Palladium(0)-catalyzed Cross-coupling Reaction of Tetra(alkoxo)diborons with Aryltriflates. Tetrahedron Lett. 1997, 38, 3447–3450. [Google Scholar]
- Willis, D. M.; Strongin, R. M. Palladium-catalyzed Borylation of Aryldiazonium Tetrafluoroborate Salts. A New Synthesis of Arylboronic Esters. Tetrahedron Lett. 2000, 41, 8683–8686. [Google Scholar]
- Letsinger, R. L.; Dandegaonker, S. H. Organoboron Compounds. IX. 8-Quinoline boronic Acid, its Preparation and Influence on Reactions of Chlorohydrins. J. Am. Chem. Soc. 1959, 81, 498–501. [Google Scholar]
- Ishiyama, T.; Miyaura, N. Chemistry of Group 13 Element-transition Metal Linkage-the Platinum and Palladium-catalyzed Reactions of (Alkoxo)diborons. J. Organomet. Chem. 2000, 611, 392–402. [Google Scholar]
- Bellamy, F. D.; Ou, K. Selective Reduction of Aromatic Nitro Compounds with Stannous Chloride in non Acidic and non Aqueous Medium. Tetrahedron Lett. 1984, 25, 839–842. [Google Scholar]
- Elderfield, R. C.; Krueger, G. L. Synthesis of Bz-Polymethoxy-8-Aminoquinolines and some Derivatives Thereof. J. Org. Chem. 1952, 17, 358–370. [Google Scholar]
- Samples Availability: Available from the authors.
© 2004 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Fang, H.; Yan, J.; Wang, B. Biaryl Product Formation from Cross-coupling in Palladiumcatalyzed Borylation of a Boc Protected Aminobromoquinoline Compound. Molecules 2004, 9, 178-184. https://doi.org/10.3390/90300178
Fang H, Yan J, Wang B. Biaryl Product Formation from Cross-coupling in Palladiumcatalyzed Borylation of a Boc Protected Aminobromoquinoline Compound. Molecules. 2004; 9(3):178-184. https://doi.org/10.3390/90300178
Chicago/Turabian StyleFang, Hao, Jun Yan, and Binghe Wang. 2004. "Biaryl Product Formation from Cross-coupling in Palladiumcatalyzed Borylation of a Boc Protected Aminobromoquinoline Compound" Molecules 9, no. 3: 178-184. https://doi.org/10.3390/90300178



