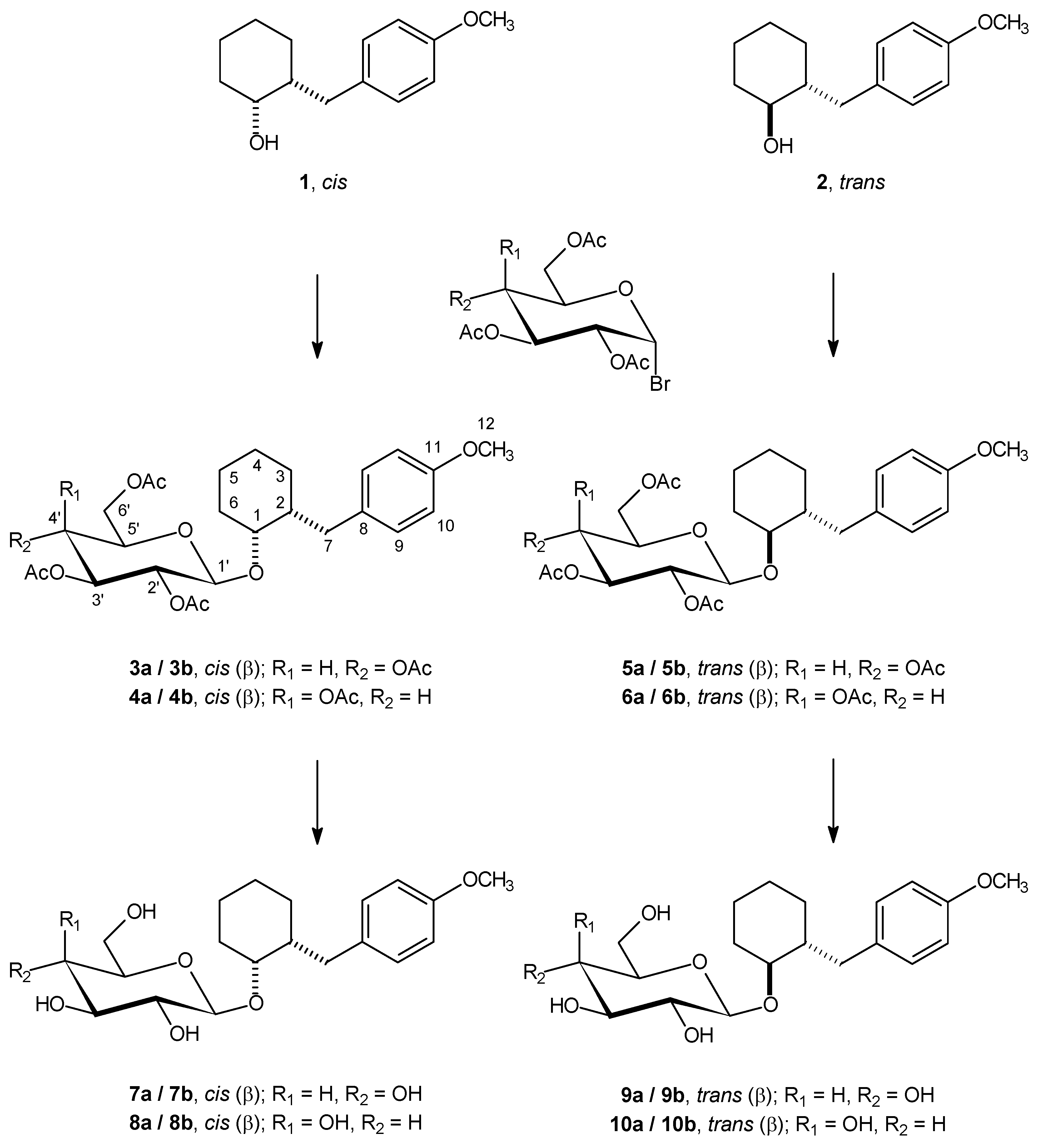
2-(4-Methoxybenzyl)cyclohexyl-2’,3’,4’,6’-tetra-O-acetyl-β-d-glucopyranoside (3a/3b and 5a/5b) and 2-(4-methoxybenzyl)cyclohexyl-2’,3’,4’,6’-tetra-O-acetyl-β-d-galactopyranoside (4a/4b and 6a/6b).
The respective isomers of 2-(4-methoxybenzyl)cyclohexanol (0.272 g; 1.24 mmol) were dissolved in toluene (30 mL), and cadmium carbonate (0.656 g; 3.72 mmol) was added. A portion of the toluene (7 to 10 mL) was distilled off under azeotropic conditions to remove all traces of water from the system. A solution of 2,3,4,6-tetra-O-acetyl-α-d-glucopyranosyl bromide or 2,3,4,6-tetra-O-acetyl-α-d-galactopyranosyl bromide (1.528 g; 3.72 mmol) in toluene (2 mL) was then added over 5 minutes to the reaction mixture, which was further stirred and heated under azeotropic conditions for an additional 6 h. A mixture of inorganic salts was removed by filtration, and the solvent was evaporated from the filtrate, affording a dry residue. Silica gel column chromatography (eluent: 3:1 to 1:1 step-wise gradient of light petroleum – ether) yielded the pure products in yields of 61.6 % (3a/3b), 61.8 % (4a/4b), 83.6 % (5a/5b) and 73.7 % (6a/6b), respectively.
3a/3b: 1H-NMR (CDCl3): Diastereoisomer A: 1.17 – 1.43 (m, 6H, H-3a, 3b, 4a, 5a, 5b and 6a), 1.57 – 1.68 (m, 2H, H-2 and 4b), 1.84 (m, 1H, H-6b), 2.05 (s, 3H, OAc), 2.06 (s, 3H, OAc), 2.08 (s, 6H, 2 × OAc), 2.41 (dd, J = 6.4, 13.8Hz, 1H, H-7a), 2.72 (dd, J = 7.8, 13.8Hz, 1H, H-7b), 3.65 (ddd, J = 2.6, 4.7, 10.0Hz, 1H, H-5’), 3.80 (s, 3H, H-12), 3.81 (dt, J = 2.4, 2.4, 4.3Hz, 1H, H-1), 4.14 (dd, J = 2.6, 12.2Hz, 1H, H-6’a), 4.28 (dd, J = 4.7, 12.2Hz, 1H, H-6’b), 4.56 (d, J = 7.9Hz, 1H, H-1’), 5.11 (dd, J = 7.9, 9.9Hz, 1H, H-2’), 5.15 (t, J = 9.8Hz, 1H, H-4’), 5.22 (t, J = 9.8Hz, 1H, H-3’), 6.77 (m, 2H, H-9), 7.13 (m, 2H, H-8); Diastereoisomer B: 1.17 – 1.43 (m, 6H, H-3a, 3b, 4a, 5a, 5b and 6a), 1.57 – 1.68 (m, 2H, H-2 and 4b), 1.84 (m, 1H, H-6b), 2.02 (s, 6H, 2 × OAc), 2.03 (s, 6H, 2 × OAc), 2.39 (dd, J = 7.7, 13.6Hz, 1H, H-7a), 2.53 (dd, J = 7.0, 13.6Hz, 1H, H-7b), 3.64 (ddd, J = 2.6, 5.5, 10.1Hz, 1H, H-5’), 3.65 (dt, J = 2.4, 2.4, 4.5Hz, 1H, H-1), 3.77 (s, 3H, H-12), 4.11 (dd, J = 2.6, 12.2Hz, 1H, H-6’a), 4.21 (dd, J = 5.5, 12.2Hz, 1H, H-6’b), 4.55 (d, J = 7.9Hz, 1H, H-1’), 5.06 (dd, J = 9.3, 10.1Hz, 1H, H-4’), 5.09 (dd, J = 7.9, 9.8Hz, 1H, H-2’), 5.23 (t, J = 9.5Hz, 1H, H-3’), 6.84 (m, 2H, H-10), 7.06 (m, 2H, H-9). IR (cm-1, CCl4): 3027 (w), 2960 (w), 2936 (w), 2836 (w), 1756 (s), 1613 (w), 1513 (m), 1441 (w), 1388 (m), 1300 (w), 1245 (s), 1220 (s), 1042 (s), 895 (w), 843 (w). MS (FAB): [M]+ 550 (4), 331 (28), 289 (5), 229 (6), 203 (24), 169 (56), 121 (100), 109 (40), 77 (8). [α]D20 = -99 (c 0.22, CHCl3). For C28H38O11 (550.61): C 61.08, H 6.96; found: C 60.92, H 6.95.
4a/4b: 1H-NMR (CDCl3): Diastereoisomer A: 0.89 (m, 1H, H-3a), 1.18 (m, 1H, H-5a), 1.22 (m, 1H, H-4a), 1.36 (m, 1H, H-6), 1.57 m, 1H, H-5b), 1.62 (m, 1H, H-2), 1.63 (m, 1H, H-3b), 1.67 (m, 1H, H-4b), 1.84 (m, 1H, H-6b), 2.00 (s, 3H, OAc), 2.02 (s, 3H, OAc), 2.09 (s, 3H, OAc), 2.17 (s, 3H, OAc), 2.44 (dd, J = 7.0, 13.8Hz, 1H, H-7a), 2.74 (dd, J = 7.4, 13.8Hz, 1H, H-7b), 3.80 (s, 3H, H-12), 3.81 (dt, J = 2.3, 2.3, 4.5Hz, 1H, H-1), 3.87 (dt, J = 1.2, 6.7, 6.7Hz, 1H, H-5’), 4.16 (d, J = 6.7Hz, 2H, H-6a, H-6b), 4.51 (d, J = 7.9Hz, 1H, H-1’), 5.05 (dd, J = 3.5, 10.5Hz, 1H, H-3’), 5.30 (dd, J = 7.9, 10.5Hz, 1H, H-2’), 5.40 (dd, J = 1.2, 3.5Hz, 1H, H-4’), 6.80 (m, 2H, H-10), 7.13 (m, 2H, H-9); Diastereoisomer B: 0.89 (m, 1H, H-3a), 1.18 (m, 1H, H-5a), 1.22 (m, 1H, H-4a), 1.36 (m, 1H, H-6), 1.57 m, 1H, H-5b), 1.62 (m, 1H, H-2), 1.63 (m, 1H, H-3b), 1.67 (m, 1H, H-4b), 1.84 (m, 1H, H-6b), 2.00 (s, 3H, OAc), 2.02 (s, 3H, OAc), 2.06 (s, 3H, OAc), 2.09 (s, 3H, OAc), 2.41 (dd, J = 8.0, 13.6Hz, 1H, H-7a), 2.54 (dd, J = 6.8, 13.6Hz, 1H, H-7b), 3.66 (dt, J = 2.6, 2.6, 4.6Hz, 1H, H-1), 3.78 (s, 3H, H-12), 3.84 (dt, J = 1.2, 6.7, 6.7Hz, 1H, H-5’), 4.08 (dd, J = 6.8, 11.2Hz, 1H, H-6’a), 4.16 (dd, J = 6.6, 11.2Hz, 1H, H-6’b), 4.50 (d, J = 7.9, 1H, H-1’), 5.04 (dd, J = 3.5, 10.5Hz, 1H, H-3’), 5.26 (dd, J = 7.9, 10.5Hz, 1H, H-2’), 5.38 (dd, J = 1.2, 3.5Hz, 1H, H-4’), 6.84 (m, 2H, H-10), 7.02 (m, 2H, H-9). IR (cm-1, CCl4): 3063 (w), 3030 (w), 2936 (m), 2859 (w), 2836 (w), 1757 (s), 1613 (w), 1513 (s), 1441 (w), 1369 (s), 1300 (w), 1246 (s), 1221 (s), 1173 (s), 1056 (s), 1045 (s), 898 (w), 842 (w). MS (FAB): [M]+ 550 (1), 331 (10), 203 (12), 169 (24), 121 (100), 109 (28), 91 (10). [α]D20 = -70 (c 0.24, CHCl3). For C28H38O11 (550.61): C 61.08, H 6.96; found: C 61.00, H 6.92.
5a/5b: 1H-NMR (CDCl3): Diastereoisomer A: 0.88 (m, 1H, H-3a), 1.10 (m, 1H, H-5a), 1.22 (m, 1H, H-4a), 1.42 (m, 1H, H-6a), 1.56 (m, 1H, H-5b), 1.58 (m, 1H, H-2), 1.63 (m, 1H, H-3b), 1.72 (m, 1H, H-4b), 2.00 (s, 3H, OAc), 2.03 (s, 3H, OAc), 2.05 (s, 3H, OAc), 2.08 (s, 3H, OAc), 2.16 (m, 1H, H-6b), 2.19 (dd, J = 9.3, 13.6Hz, 1H, H-7a), 3.13 (dd, J = 3.6, 13.6Hz, 1H, H-7b), 3.32 (dt, J = 4.2, 9.5, 9.5Hz, 1H, H-1), 3.71 (ddd, J = 2.6, 5.4, 10.1Hz, 1H, H-5’), 3.78 (s, 3H, H-12), 4.17 (dd, J = 2.6, 12.1Hz, 1H, H-6’a), 4.27 (dd, J = 5.4, 12.1Hz, 1H, H-6’b), 4.63 (d, J = 8.0, 1H, H-1’), 5.08 (dd, J = 8.0, 9.6Hz, 1H, H-2’), 5.11 (t, J = 9.8Hz, 1H, H-4’), 5.22 (t, J = 9.6Hz, 1H, H-3’), 6.81 (m, 2H), 7.09 (m, 2H); Diastereoisomer B: 0.88 (m, 1H, H-3a), 1.10 (m, 1H, H-5a), 1.22 (m, 1H, H-4a), 1.42 (m, 1H, H-6a), 1.56 (m, 1H, H-5b), 1.58 (m, 1H, H-2), 1.63 (m, 1H, H-3b), 1.72 (m, 1H, H-4b), 2.00 (s, 3H, OAc), 2.03 (s, 3H, OAc), 2.05 (s, 3H, OAc), 2.08 (s, 3H, OAc), 2.09 (dd, J = 8.9, 13.2Hz, 1H, H-7a), 3.07 (dd, J = 3.3, 13.2Hz, 1H, H-7b), 3.21 (dt, J = 4.3, 9.7, 9.7Hz, 1H, H-1), 3.68 (ddd, J = 2.7, 4.7, 10.0Hz, 1H, H-5’), 3.78 (s, 3H, H-12), 4.13 (dd, J = 2.7, 12.1Hz, 1H, H-6’a), 4.25 (dd, J = 4.7, 12.1Hz, 1H, H-6’b), 4.62 (d, J = 8.0, 1H, H-1’), 5.01 (dd, J = 8.0, 9.8Hz, 1H, H-2’), 5.10 (t, J = 9.8Hz, 1H, H-4’), 5.22 (t, J = 9.6Hz, 1H, H-3’), 6.80 (m, 2H, H-10), 7.02 (m, 2H, H-9). IR (cm-1, CCl4): 3031 (w), 2935 (m), 2859 (w), 2853 (w), 1761 (s), 1753 (s), 1613 (w), 1513 (m), 1442 (w), 1300 (w), 1246 (s), 1225 (s), 1040 (s), 880 (w), 856 (w). MS (FAB): [M]+ 550 (2), 331 (25), 289 (10), 229 (8), 202 (14), 169 (62), 121 (100), 109 (46), 91 (16). [α]D20 = -103 (c 0.25, CHCl3). For C28H38O11 (550.61): C 61.08, H 6.96; found: C 61.05, H 6.98.
6a/6b: 1H-NMR (CDCl3): Diastereoisomer A: 0.88 (m, 1H, H-3a), 1.06 (m, 1H, H-5a), 1.19 (m, 1H, H-6a), 1.20 (m, 1H, H-4a), 1.52 (m, 1H, H-5b), 1.58 (m, 1H, H-2), 1.64 (m, 1H, H-3b), 1.71 (m, 1H, H-4b), 1.95 (m, 1H, H-6b), 2.09 (s, 3H), 2.09-2.14 (m, 4H, H-7a, OAc), 2.12 (s, 3H, OAc), 2.14 (s, 3H, OAc), 2.16 (s, 3H, OAc), 3.19 (dd, J = 3.8, 13.4Hz, 1H, H-7b), 3.32 (dt, J = 4.1, 9.5, 9.5Hz, 1H, H-1), 3.78 (s, 3H, H-12), 3.92 (dt, J = 1.3, 5.8, 6.8Hz, H-5’), 4.10 (dd, J = 5.8, 11.2Hz, 1H, H-6’a), 4.22 (dd, J = 6.8, 11.2Hz, 1H, H-6’b), 4.60 (d, J = 8.0Hz, 1H, H-1’), 5.04 (dd, J = 3.5, 10.5Hz, 1H, H-3’), 5.31 (dd, J = 8.0, 10.5Hz, 1H, H-2’), 5.39 (dd, J = 1.3, 3.5Hz, 1H, H-4’), 6.82 (m, 2H, H-10), 7.09 (m, 2H, H-9); Diastereoisomer B: 0.88 (m, 1H, H-3a), 1.06 (m, 1H, H-5a), 1.19 (m, 1H, H-6a), 1.20 (m, 1H, H-4a), 1.52 (m, 1H, H-5b), 1.58 (m, 1H, H-2), 1.64 (m, 1H, H-3b), 1.71 (m, 1H, H-4b), 1.95 (m, 1H, H-6b), 2.03 (s, 3H, OAc), 2.03-2.06 (m, 1H, H-7a), 2.04 (s, 3H, OAc), 2.05 (s, 3H, OAc), 2.06 (s, 3H, OAc), 3.08 (dd, J = 3.3, 13.2Hz, 1H, H-7b), 3.22 (dt, J = 4.3, 9.9, 9.9Hz, 1H, H-1), 3.78 (s, 3H, H-12), 3.89 (dt, J = 1.3, 6.3, 6.3Hz, 1H, H-5’), 4.11 (dd, J = 6.2, 11.1Hz, 1H, H-6’a), 4.19 (dd, J = 6.3, 11.1Hz, 1H, H-6’b), 4.56 (d, J = 7.9Hz, 1H, H-1’), 5.04 (dd, J = 3.5, 10.5Hz, 1H, H-3’), 5.20 (dd, J = 7.9, 10.5Hz, 1H, H-2’), 5.39 (dd, J = 1.3, 3.5Hz, 1H, H-4’), 6.80 (m, 2H, H-10), 7.03 (m, 2H, H-9). IR (cm-1, CCl4): 3063 (w), 3031 (w), 2935 (m), 2859 (m), 2836 (w), 1757 (s), 1613 (w), 1513 (s), 1441 (w), 1369 (s), 1300 (w), 1245 (s), 1221 (s), 1172 (s), 1044 (s), 880 (w), 856 (w). MS (FAB): [M]+ 550 (1), 331 (16), 202 (18), 169 (36), 121 (100), 109 (34), 81 (10). [α]D20 = -72 (c 0.21, CHCl3). For C28H38O11 (550.61): C 61.08, H 6.96; found: C 60.99, H 6.99.
2-(4-Methoxybenzyl)cyclohexyl-β-d-glucopyranoside (7a/7b and 9a/9b) and 2-(4-methoxybenzyl)-cyclohexyl-β-d-galactopyranoside (8a/8b and 10a/10b).
A solution of the respective compounds 3a/3b – 6a/6b (0.206 g; 0.374 mmol) in a mixture of methanol (20 mL) and water (4 mL) was heated to reflux in the presence of potassium carbonate (0.3 g) for 2 h. Methanol and water were removed under reduced pressure, and the residue was applied on the top of a column filled with silica gel and purified by elution with a 15:1 to 5:1 stepwise gradient of chloroform / methanol mixture, affording the pure products in yields of 92.4 % (7a/7b), 88.6 % (8a/8b), 53.8 % (9a/9b) and 66.4 % (10a/10b), respectively.
7a/7b: 1H-NMR (CD3OD): Diastereoisomer A: 1.24 (m, 1H, H-4a), 1.32 (m, 1H, H-3a), 1.41 (m, 2H, H-5a, H-6a), 1.52 (m, 1H, H-3b), 1.65 (m, 1H, H-4b), 1.72 (m, 1H, H-5b), 1.76 (m, 1H, H-2), 2.04 (m, 1H, H-6b), 2.52 (dd, J = 8.4, 13.7Hz, 1H, H-7a), 2.84 (dd, J = 6.6, 13.7Hz, 1H, H-7b), 3.25 (ddd, J = 2.5, 5.6, 9.7Hz, 1H, H-5’), 3.27 (dd, J = 7.7, 9.2Hz, 1H, H-2’), 3.32 (t, J = 9.5Hz, 1H, H-4’), 3.37 (t, J = 9.2Hz, 1H, H-3’), 3.72 (dd, J = 5.6, 11.7Hz, 1H, H-6’a), 3.75 (s, 3H, H-12), 3.89 (dd, J = 2.5, 11.7Hz, 1H, H-6’b), 3.92 (dt, J = 2.5, 2.5, 4.9Hz, 1H, H-1), 4.34 (d, J = 7.7Hz, 1H, H-1’), 6.79 (m, 2H, H-10), 7.15 (m, 2H, H-9); Diastereoisomer B: 1.24 (m, 1H, H-4a), 1.32 (m, 2H, H-3a, H-6a), 1.36 (m, 1H, H-5a), 1.52 (m, 1H, H-3b), 1.65 (m, 1H, H-4b), 1.70 (m, 1H, H-2), 1.77 (m, 1H, H-5b), 1.96 (m, 1H, H-6b), 2.42 (dd, J = 7.9, 13.6Hz, 1H, H-7a), 2.81 (dd, J = 6.5, 13.6Hz, 1H, H-7b), 3.20 (ddd, J = 2.4, 5.7, 9.6Hz, 1H, H-5’), 3.25 (dd, J = 7.8, 9.1Hz, 1H, H-2’), 3.27 (t, J = 9.7Hz, 1H, H-4’), 3.35 (dd, J = 9.2, 9.8Hz, 1H, H-3’), 3.65 (dd, J = 5.7, 11.8Hz, 1H, H-6’a), 3.73 (dt, J = 2.4, 2.4, 4.8Hz, 1H, H-1), 3.75 (s, 3H, H-12), 3.82 (dd, J = 2.4, 11.8Hz, 1H, H-6’b), 4.33 (d, J = 7.8Hz, 1H, H-1’), 6.80 (m, 2H, H-10), 7.15 (m, 2H, H-9). IR (cm-1, KBr): 3401 (s), 2994 (w), 2932 (s), 1612 (m), 1513 (s), 1177 (s), 1096 (s), 1074 (s), 1036 (s), 1018 (s), 895 (w), 842 (w). MS (FAB): [M+H]+ 383 (1), 325 (1), 273 (1), 261 (1), 241 (1), 203 (16), 121 (100), 91 (5), 79 (7). [α]D20 = -63 (c 0.19, CH3OH). For C20H30O7 (382.46): C 62.81, H 7.91; found: C 62.75, H 7.92.
8a/8b: 1H-NMR (CD3OD): Diastereoisomer A: 1.25 (m, 1H, H-4a), 1.33 (m, 1H, 3a), 1.37 (m, 1H, H-5a), 1.41 (m, 1H, H-6a), 1.52 (m, 1H, H-3b), 1.66 (m, 1H, H-4b), 1.72 (m, 1H, H-5b), 1.76 (m, 1H, H-2), 2.02 (m, 1H, H-6b), 2.52 (dd, J = 8.4, 13.6Hz, 1H, H-7a), 2.85 (dd, J = 6.6, 13.6Hz, 1H, H-7b), 3.48 (dd, J = 3.5, 9.7Hz, 1H, H-3’), 3.48 (dt, J = 1.2, 6.2, 6.2Hz, 1H, H-5’), 3.60 (dd, J = 7.7, 9.7Hz, 1H, H-2’), 3.75 (s, 3H, H-12), 3.76 (dd, J = 6.1, 11.0Hz, 1H, H-6’a), 3.80 (dd, J = 6.3, 11.0Hz, 1H, H-6’b), 3.88 (dt, J = 1.2, 3.5Hz, 1H, H-4’), 3.91 (dt, J = 2.4, 2.4, 4.9Hz, 1H, H-1), 4.30 (d, J = 7.7Hz, 1H, H-1’), 6.79 (m, 2H, H-10), 7.15 (m, 2H, H-9); Diastereoisomer B: 1.25 (m, 1H, H-4a), 1.31 (m, 1H, H-6a), 1.33 (m, 1H, H-3a), 1.35 (m, 1H, H-5a), 1.52 (m, 1H, H-3b), 1.66 (m, 1H, H-4b), 1.69 (m, 1H, H-2), 1.78 (m, 1H, H-5b), 1.96 (m, 1H, H-6b), 2.42 (dd, J = 7.8, 13.7Hz, 1H, H-7a), 2.81 (dd, J = 6.6, 13.7Hz, 1H, H-7b), 3.44 (dt, J = 1.2, 6.2, 6.2Hz, 1H, H-5’), 3.48 (dd, J = 3.4, 9.7Hz, 1H, H-3’), 3.58 (dd, J = 7.8, 9.7Hz, 1H, H-2’), 3.69 (dd, J = 6.4, 11.1Hz, 1H, H-6’a), 3.72 (dd, J = 6.1, 11.1Hz, 1H, H-6’b), 3.74 (dt, J = 2.4, 2.4, 4.8Hz, 1H, H-1), 3.75 (s, 3H, H-12), 3.84 (dd, J = 1.1, 3.4Hz, 1H, H-4’), 4.28 (d, J = 7.8Hz, 1H, H-1’), 6.80 (m, 2H, H-10), 7.14 (m, 2H, H-9). IR (cm-1, KBr): 3413 (s), 2923 (s), 2908 (m), 1611 (w), 1512 (s) 1105 (m), 1090 (s), 1082 (s), 1049 (s), 1038 (s), 848 (w). MS (FAB): [M+H]+ 383 (1), 231 (2), 203 (9), 121 (100), 91 (8), 79 (17). [α]D20 = -82 (c 0.20, CH3OH). For C20H30O7 (382.46): C 62.81, H 7.91; found: C 62.87, H 7.85.
9a/9b: 1H-NMR (CD3OD): Diastereoisomer A: 0.90 (m, 1H, H-3a), 1.08 (m, 1H, H-5a), 1.22 (m, 1H, H-6a), 1.24 (m, 1H, H-4a), 1.55 (m, 1H, H-5b), 1.58 (m, 1H, H-2), 1.60 (m, 1H, H-3b), 1.73 (m, 1H, H-4b), 2.13 (m, 1H, H-6b), 2.22 (dd, J = 9.4, 13.7Hz, 1H, H-7a), 3.23 – 3.41 (m, 3H, H-3’, 4’ and 5’), 3.24 (dd, J = 7.8, 9.2Hz, 1H, H-2’), 3.39 (dd, J = 2.9, 13.7Hz, 1H, H-7b), 3.49 (dt, J = 4.1, 9.6, 9.6Hz, 1H, H-1), 3.70 (dd, J = 5.6, 11.7Hz, 1H, H-6’a), 3.75 (s, 3H, H-12), 3.90 (dd, J = 2.3, 11.7Hz, 1H, H-6’b), 4.41 (d, J = 7.8Hz, 1H, H-1’), 6.80 (m, 2H, H-10), 7.09 (m, 2H, H-9); Diastereoisomer B: 0.90 (m, 1H, H-3a), 1.08 (m, 1H, H-5a), 1.22 (m, 1H, H-6a), 1.24 (m, 1H, H-4a), 1.55 (m, 1H, H-5b), 1.58 (m, 1H, H-2), 1.60 (m, 1H, H-3b), 1.73 (m, 1H, H-4b), 2.13 (m, 1H, H-6b), 2.15 (dd, J = 10.0, 13.3Hz, 1H, H-7a), 3.22 (dd, J = 7.8, 9.2, 1H, H-2’), 3.23 – 3.41 (m, 4H, H-7b, H-3’, H-4’ and H-5’), 3.68 (dd, J = 5.2, 11.6Hz, 1H, H-6’a), 3.75 (s, 3H, H-12), 3.85 (dd, J = 2.2, 11.7Hz, 1H, H-6’b), 4.40 (d, J = 7.8Hz, 1H, H-1’), 6.80 (m, 2H, H-10), 7.08 (m, 2H, H-9). IR (cm-1, KBr): 3429 (s), 2995 (w), 2927 (m), 1612 (m), 1513 (m), 1099 (m), 1074 (m), 1036 (s), 1024 (s), 879 (w). MS (FAB): [M+H]+ 383 (1), 261 (1), 241 (2), 203 (8), 121 (100), 91 (5), 79 (16). [α]D20 = -67 (c 0.26, CH3OH). For C20H30O7 (382.46): C 62.81, H 7.91; found: C 62.85, H 7.88.
10a/10b: 1H-NMR (CD3OD): Diastereoisomer A: 0.89 (m, 1H, H-3a), 1.08 (m, 1H, H-5a), 1.23 (m, 1H, H-4a), 1.37 (m, 1H, H-6a), 1.56 (m, 1H, 5b), 1.61 (m, 2H, H-2, H-3b), 1.72 (m, 1H, 4b), 2.14 (dd, J = 9.9, 13.4Hz, 1H, H-7a), 2.22 (m, 1H, H-6b), 3.40 (dd, J = 3.3, 13.4Hz, 1H), 3.48 (m, 1H), 3.48 (dd, J = 3.3, 9.7Hz, 1H), 3.50 (dt, J = 1.1, 6.2, 6.2Hz, 1H), 3.55 (dd, J = 7.5, 9.7Hz, 1H), 3.75 (s, 3H), 3.75 (dd, J = 6.2, 11.2Hz, 1H), 3.78 (dd, J = 6.3, 11.2Hz, 1H), 3.86 (dd, J = 1.1, 3.3Hz, 1H), 4.36 (d, J = 7.5Hz, 1H), 6.79 (m, 2H), 7.09 (m, 2H); Diastereoisomer B: 0.89 (m, 1H, H-3a), 1.08 (m, 1H, H-5a), 1.23 (m, 1H, H-4a), 1.28 (m, 1H, H-6a), 1.56 (m, 1H, 5b), 1.57 (m, 1H, H-2), 1.61 (m, 1H, H-3b), 1.72 (m, 1H, 4b), 2.14 (m, 1H, H-6b), 2.21 (dd, J = 9.5, 13.7Hz, 1H, H-7a), 3.24 (dd, J = 3.6, 13.7Hz, 1H, H-7b), 3.32 (m, 1H, H-1), 3.47 (dd, J = 3.4, 9.7Hz, 1H, H-3’), 3.50 (dt, J = 1.0, 6.2, 6.2Hz, 1H, H-5’), 3.57 (dd, J = 7.7, 9.7Hz, 1H, H-2’), 3.72 (dd, J = 6.1, 11.2Hz, 1H, H-6’a), 3.75 (s, 3H, H-12), 3.75 (dd, J = 6.3, 11.2Hz, 1H, H-6’b), 3.85 (dd, J = 1.0, 3.4Hz, 1H, H-4’), 4.36 (d, J = 7.7Hz, 1H, H-1’), 6.80 (m, 2H, H-10), 7.08 (m, 2H, H-9). IR (cm-1, KBr): 3520 (s), 3420 (s), 2990 (w), 1613 (m), 1514 (s) 1100 (m), 1084 (s), 1078 (m), 1052 (s), 1035 (s), 880 (w). MS (FAB): [M+H]+ 383 (1), 231 (1), 203 (10), 121 (100), 91 (8), 79 (11). [α]D20 = -86 (c 0.25, CH3OH). For C20H30O7 (382.46): C 62.81, H 7.91; found: C 62.78, H 7.87.
Table 1.
13C-NMR data of the protected alkyl glycosides 3a – 6b (measured in CDCl3)
Table 1.
13C-NMR data of the protected alkyl glycosides 3a – 6b (measured in CDCl3)
| Carbon atom No. | Compound |
|---|
| 3a a | 3b a | 4a b | 4b b | 5a c | 5b c | 6a d | 6b d |
|---|
| 1 | 74.65 (d) | 79.93 (d) | 75.18 (d) | 80.07 (d) | 80.94 (d) | 85.60 (d) | 81.25 (d) | 85.77 (d) |
| 2 | 43.89 (d) | 43.80 (d) | 43.81 (d) | 43.72 (d) | 45.14 (d) | 44.32 (d) | 45.12 (d) | 44.28 (d) |
| 3 | 26.82 (t) | 26.68 (t) | 26.73 (t) | 26.62 (t) | 29.76 (t) | 29.68 (t) | 29.76 (t) | 29.60 (t) |
| 4 | 25.37 (t) | 25.06 (t) | 25.30 (t) | 24.96 (t) | 24.76 (t) | 24.43 (t) | 24.76 (t) | 24.41 (t) |
| 5 | 20.76 (t) | 20.70 (t) | 20.98 (t) | 20.84 (t) | 24.91 (t) | 24.88 (t) | 24.93 (t) | 24.81 (t) |
| 6 | 31.93 (t) | 28.78 (t) | 31.93 (t) | 28.88 (t) | 33.72 (t) | 31.23 (t) | 33.79 (t) | 31.26 (t) |
| 7 | 37.23 (t) | 37.17 (t) | 37.22 (t) | 37.03 (t) | 37.30 (t) | 37.26 (t) | 37.34 (t) | 37.31 (t) |
| 8 | 133.43 (s) | 133.01 (s) | 133.51 (s) | 133.04 (s) | 132.91 (s) | 132.44 (s) | 132.99 (s) | 132.47 (s) |
| 9 | 130.30 (d) | 129.84 (d) | 130.27 (d) | 129.84 (d) | 130.40 (d) | 130.08 (d) | 130.35 (d) | 130.10 (d) |
| 10 | 113.78 (d) | 113.34 (d) | 113.77 (d) | 113.40 (d) | 113.64 (d) | 113.41 (d) | 113.64 (d) | 113.44 (d) |
| 11 | 157.83 (s) | 157.67 (s) | 157.83 (s) | 157.65 (s) | 157.81 (s) | 157.66 (s) | 157.80 (s) | 157.66 (s) |
| 12 | 55.24 (q) | 55.19 (q) | 55.24 (q) | 55.21 (q) | 55.21 (q) | 55.21 (q) | 55.21 (q) | 55.21 (q) |
| 1’ | 101.76 (d) | 98.13 (d) | 102.32 (d) | 98.85 (d) | 102.04 (d) | 98.49 (d) | 102.56 (d) | 99.24 (d) |
| 2’ | 71.76 (d) | 7159 (d) | 69.34 (d) | 69.19 (d) | 71.67 (d) | 7158 (d) | 69.32 (d) | 69.25 (d) |
| 3’ | 73.11 (d) | 73.08 (d) | 71.17 (d) | 71.16 (d) | 73.09 (d) | 73.05 (d) | 71.18 (d) | 71.18 (d) |
| 4’ | 68.94 (d) | 68.67 (d) | 67.21 (d) | 67.19 (d) | 68.77 (d) | 68.75 (d) | 67.13 (d) | 67.13 (d) |
| 5’ | 71.59 (d) | 71.52 (d) | 70.48 (d) | 70.45 (d) | 71.79 (d) | 71.60 (d) | 70.52 (d) | 70.46 (d) |
| 6’ | 62.28 (t) | 62.10 (t) | 61.37 (t) | 61.36 (t) | 62.27 (t) | 62.10 (t) | 61.36 (t) | 61.36 (t) |
Signals of the protecting acetate functionalities on the carbohydrate part of the molecules: a 20.60 (q), 20.60 (q), 20.64 (q), 20.64 (q), 20.70 (q), 20.70 (q), 20.76 (q), 20.84 (q), 169.20 (s), 169.23 (s), 169.43 (s), 169.45 (s), 170.37 (s), 170.40 (s), 170.59 (s), 170.76 (s); b 20.61 (q), 20.62 (q), 20.62 (q), 20.66 (q), 20.71 (q), 20.71 (q), 20.73 (q), 20.90 (q), 169.95 (s), 170.21 (s), 170.26 (s), 170.39 (s), 170.39 (s), 170.40 (s), 170.43 (s), 170.48 (s); c 20.60 (q), 20.60 (q), 20.60 (q), 20.63 (q), 20.69 (q), 20.69 (q), 20.69 (q), 20.71 (q), 169.27 (s), 169.32 (s), 169.41 (s), 169.42 (s), 170.35 (s), 170.37 (s), 170.62 (s), 170.68 (s); d 20.60 (q), 20.62 (q), 20.64 (q), 20.69 (q), 20.70 (q), 20.70 (q), 20.80 (q), 20.83 (q), 169.40 (s), 169.86 (s), 169.95 (s), 170.22 (s), 170.35 (s), 170.38 (s), 170.40 (s), 170.48 (s).
Table 2.
13C-NMR data of the alkyl glycosides 7a – 10b (measured in CD3OD)
Table 2.
13C-NMR data of the alkyl glycosides 7a – 10b (measured in CD3OD)
| Carbon atom No. | Compound |
|---|
| 7a | 7b | 8a | 8b | 9a | 9b | 10a | 10b |
|---|
| 1 | 76.58 (d) | 80.54 (d) | 76.48 (d) | 80.55 (d) | 81.22 (d) | 85.90 (d) | 81.26 (d) | 85.84 (d) |
| 2 | 45.61 (d) | 45.24 (d) | 45.71 (d) | 45.33 (d) | 47.44 (d) | 46.72 (d) | 47.46 (d) | 46.73 (d) |
| 3 | 28.30 (t) | 28.23 (t) | 28.30 (t) | 28.27 (t) | 31.57 (t) | 31.38 (t) | 31.58 (t) | 31.39 (t) |
| 4 | 26.59 (t) | 26.09 (t) | 26.55 (t) | 26.14 (t) | 26.43 (t) | 26.21 (t) | 26.43 (t) | 26.22 (t) |
| 5 | 23.30 (t) | 22.34 (t) | 23.28 (t) | 22.37 (t) | 26.85 (t) | 26.70 (t) | 26.86 (t) | 26.71 (t) |
| 6 | 33.25 (t) | 30.13 (t) | 33.29 (t) | 30.18 (t) | 35.71 (t) | 32.73 (t) | 35.75 (t) | 32.78 (t) |
| 7 | 38.36 (t) | 37.41 (t) | 38.25 (t) | 37.46 (t) | 39.03 (t) | 38.99 (t) | 39.01 (t) | 39.01 (t) |
| 8 | 135.61 (s) | 135.44 (s) | 135.65 (s) | 135.49 (s) | 135.14 (s) | 134.95 (s) | 135.13 (s) | 134.99 (s) |
| 9 | 131.79 (d) | 131.69 (d) | 131.81 (d) | 131.70 (d) | 131.84 (d) | 131.76 (d) | 131.86 (d) | 131.76 (d) |
| 10 | 115.04 (d) | 114.95 (d) | 115.03 (d) | 114.93 (d) | 114.99 (d) | 114.95 (d) | 114.98 (d) | 114.93 (d) |
| 11 | 159.70 (s) | 159.64 (s) | 159.68 (s) | 159.62 (s) | 159.70 (s) | 159.66 (s) | 159.69 (s) | 159.65 (s) |
| 12 | 56.12 (q) | 56.12 (q) | 56.12 (q) | 56.12 (q) | 56.12 (q) | 56.12 (q) | 56.12 (q) | 56.12 (q) |
| 1’ | 105.66 (d) | 102.23 (d) | 106.32 (d) | 102.98 (d) | 106.20 (d) | 101.81 (d) | 106.83 (d) | 102.54 (d) |
| 2’ | 76.08 (d) | 75.77 (d) | 73.54 (d) | 73.24 (d) | 76.13 (d) | 75.68 (d) | 73.57 (d) | 73.13 (d) |
| 3’ | 78.83 (d) | 78.83 (d) | 75.74 (d) | 75.70 (d) | 78.76 (d) | 78.74 (d) | 75.66 (d) | 75.64 (d) |
| 4’ | 72.39 (d) | 72.29 (d) | 70.75 (d) | 70.75 (d) | 72.42 (d) | 72.21 (d) | 70.81 (d) | 70.75 (d) |
| 5’ | 78.29 (d) | 78.22 (d) | 76.90 (d) | 76.86 (d) | 78.41 (d) | 78.28 (d) | 77.01 (d) | 76.92 (d) |
| 6’ | 63.47 (t) | 63.35 (t) | 62.83 (t) | 62.83 (t) | 63.52 (t) | 63.31 (t) | 62.92 (t) | 62.87 (t) |