Introduction
Early investigations into the potentially useful pharmacological properties of chroman and benzodipyran derivatives focused primarily on their antiallergic [
1] and antimicrobial [
2] activities. A resurgence of interest in these compounds since the early 1990s explored their utility across a broad spectrum of activities such as anticancer agents with low toxicity [
4], anti-inflammatory agents [
5], potassium channel openers with possible cardioprotective properties [
6,
7], and agonists at serotonergic 5-HT
1A receptors [
8].
It is therefore not surprising that the EI mass spectrometry (EIMS) of chroman derivatives has been studied extensively [
9,
10,
11,
12,
13,
14]. Due to earlier work [
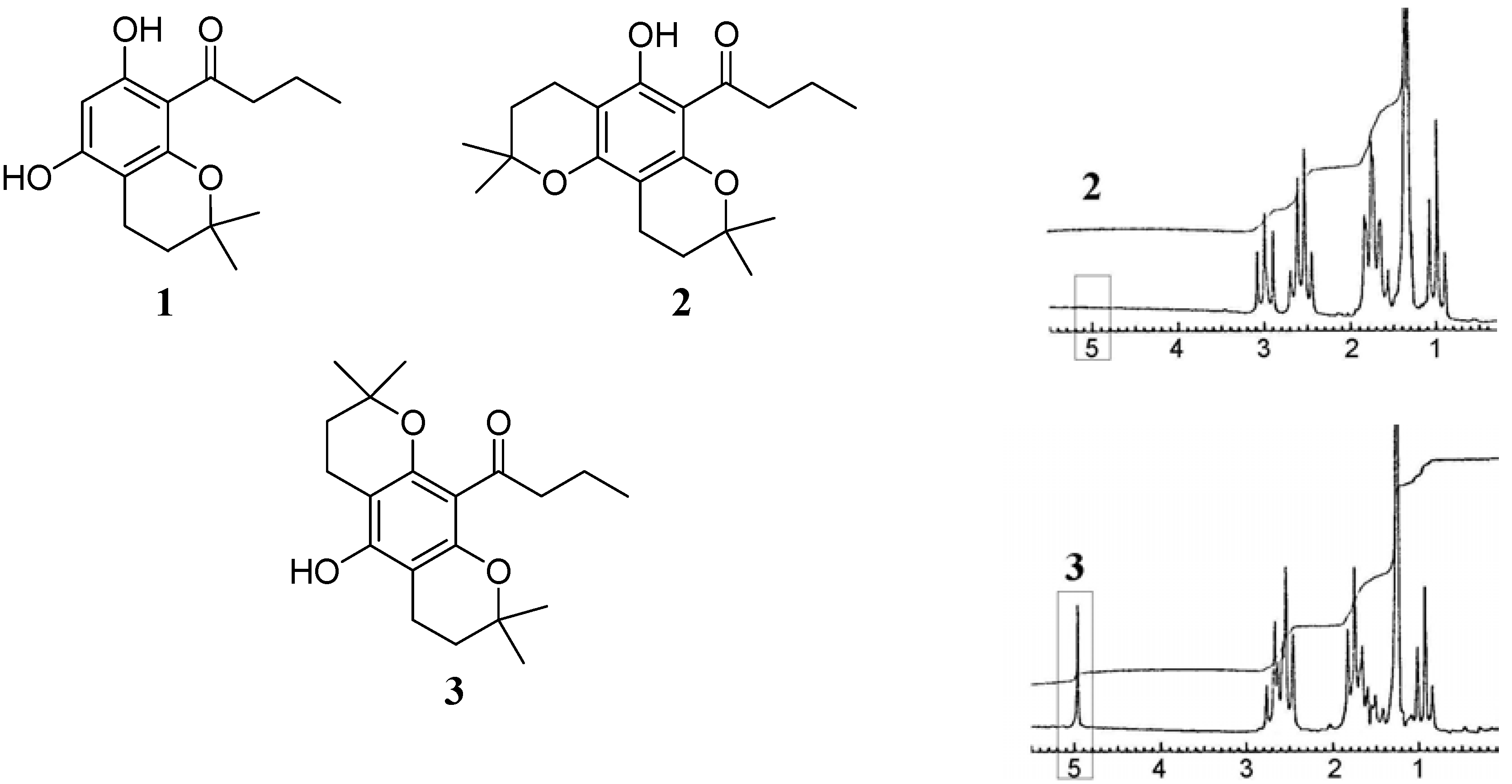
2] with the 2,2-dimethylchroman (5,7-dihydroxy-2,2-dimethyl-8-butyryl chroman)
1 and benzodipyran derivatives
2 and
3 (
Figure 1), the mass spectrometry of related compounds was of particular interest to us. We proceeded to study these compounds with simple EIMS and low resolution NMR.
Figure 1.
Structures of compounds discussed in the text: 5,7-dihydroxy-2,2- dimethyl-8-butyryl chroman (1), the "angular" benzodipyran isomer 2 and the "linear" benzodipyran isomer 3; (right) 80 MHz NMR spectra of 2 and 3 respectively, showing the exchangeable phenolic proton signal at 4.95 ppm in the case of 3.
Figure 1.
Structures of compounds discussed in the text: 5,7-dihydroxy-2,2- dimethyl-8-butyryl chroman (1), the "angular" benzodipyran isomer 2 and the "linear" benzodipyran isomer 3; (right) 80 MHz NMR spectra of 2 and 3 respectively, showing the exchangeable phenolic proton signal at 4.95 ppm in the case of 3.
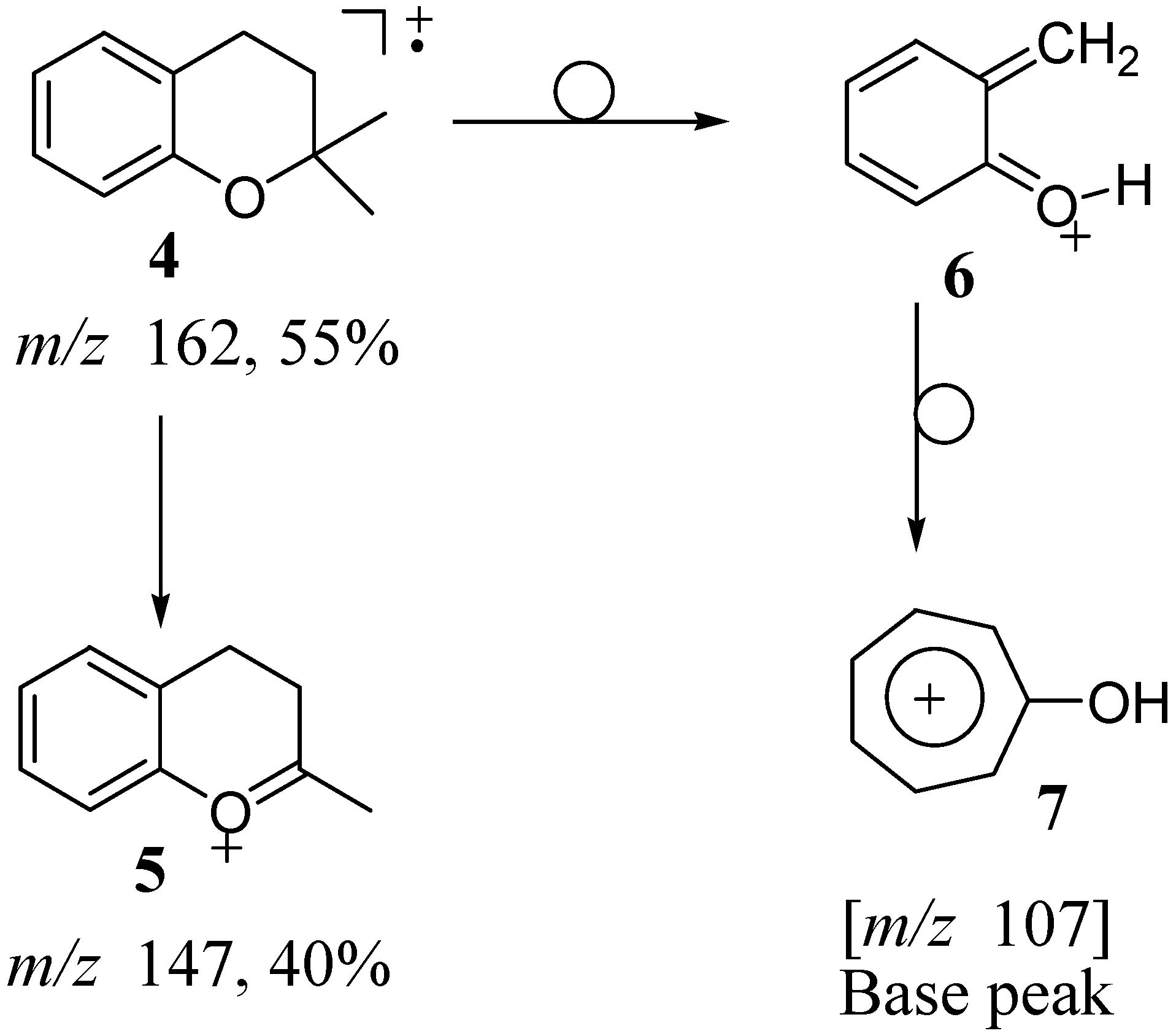
The EIMS of 2,2-dimethylchroman
4 (
Scheme 1) was shown [
9,
10] to proceed via two main fragmentation pathways. The first pathway involved the loss of a methyl group (major fragment) followed by either the loss of ethylene or the second methyl group (minor fragments). The fragment ion
5 at
m/z 147 (corresponding to the loss of a methyl group) is prominent (40% intensity) in the EI mass spectrum (
Scheme 1). The other fragmentation pathway proceeds via loss of a [CH
2=C(CH
3)CH
2] fragment (55 amu) and is accompanied by transfer of a hydrogen to the oxygen atom, giving rise to the base peak
6 at
m/z 107. The protonated quinone methide intermediate
6 rearranges to a more stable tropylium ion
7. The details of the fragmentation as well as the origins of the carbons and the hydrogens in the fragment ions were elegantly elucidated by Trudell
et al. [
10] through analogs regioselectively labeled with deuterium and
13C. Further discussions detailing these aspects will not be elaborated within this report. Rather, the focus of this paper will be to offer explanations for the striking differences observed in the fragmentation patterns reported for 2,2-dimethylchroman
4 and those of the three chroman derivatives
1,
2 and
3 synthesized by us (see
Figure 1). Additionally, a rational explanation is presented for the drastic differences in the fragmentation patterns of the two benzodipyrans, the "linear" and "angular" isomers,
2 and
3, respectively.
Scheme 1.
EI fragmentation of 2,2-dimethylchroman 4 (intensities are indicated in % relative to the base peak).
Scheme 1.
EI fragmentation of 2,2-dimethylchroman 4 (intensities are indicated in % relative to the base peak).
Results and Discussion
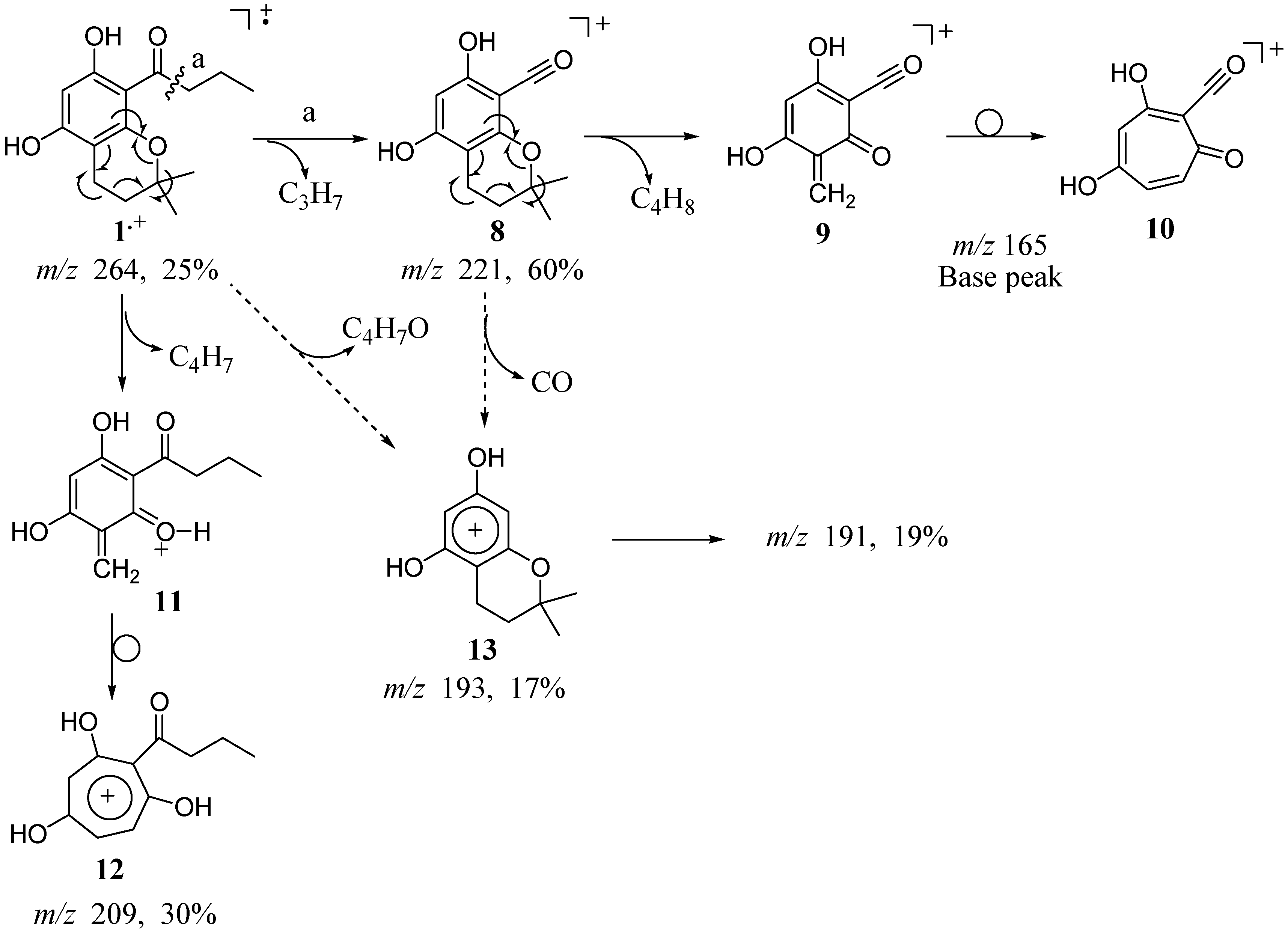
The monochroman, 5,7-dihydroxy-2,2-dimethyl-8-butyryl chroman (
1) exhibits two major fragmentation pathways (
Scheme 2). The first proceeds via cleavage that occurs β to the aromatic moiety yielding molecular ion
8 at
m/z 221 corresponding to the loss of the propyl group (43 amu) (
Figure 2) followed by a retro Diels-Alder rearrangement of the chroman moiety to give the quinone methide ion
9 at
m/z 165. This intermediate most likely rearranges to a more stable oxotropylium ion
10, as previously described for
4 [
10]. The stable oxotropylium ion constitutes the base peak of the spectrum. The retro Diels-Alder rearrangement occurs without hydrogen transfer to the oxygen atom, and thus results in the loss of the [CH
2=C(CH
3)
2] fragment (56 amu). The second major pathway proceeds by fragmentation of the chroman with hydrogen transfer to the oxygen and loss of a fragment of 55 amu. This loss yields a fragment ion
11 at
m/z 209, which likely rearranges to the more stable tropylium ion
12. Cleavage α to the aromatic ring and loss of a [CO-propyl] fragment to yield the chromandiol species
13 at
m/z 193 (17% intensity) is also observed. However, it was not determined whether this loss occurs stepwise from ion
8 or directly from the parent ion
1.+. Of interest is that in contrast to the fragmentation pattern reported for
4, no loss of a methyl group from the chroman moiety is observed in this spectrum.
Scheme 2.
EI fragmentation of 5,7-dihydroxy-2,2-dimethyl-8-butyryl chroman (1).
Scheme 2.
EI fragmentation of 5,7-dihydroxy-2,2-dimethyl-8-butyryl chroman (1).
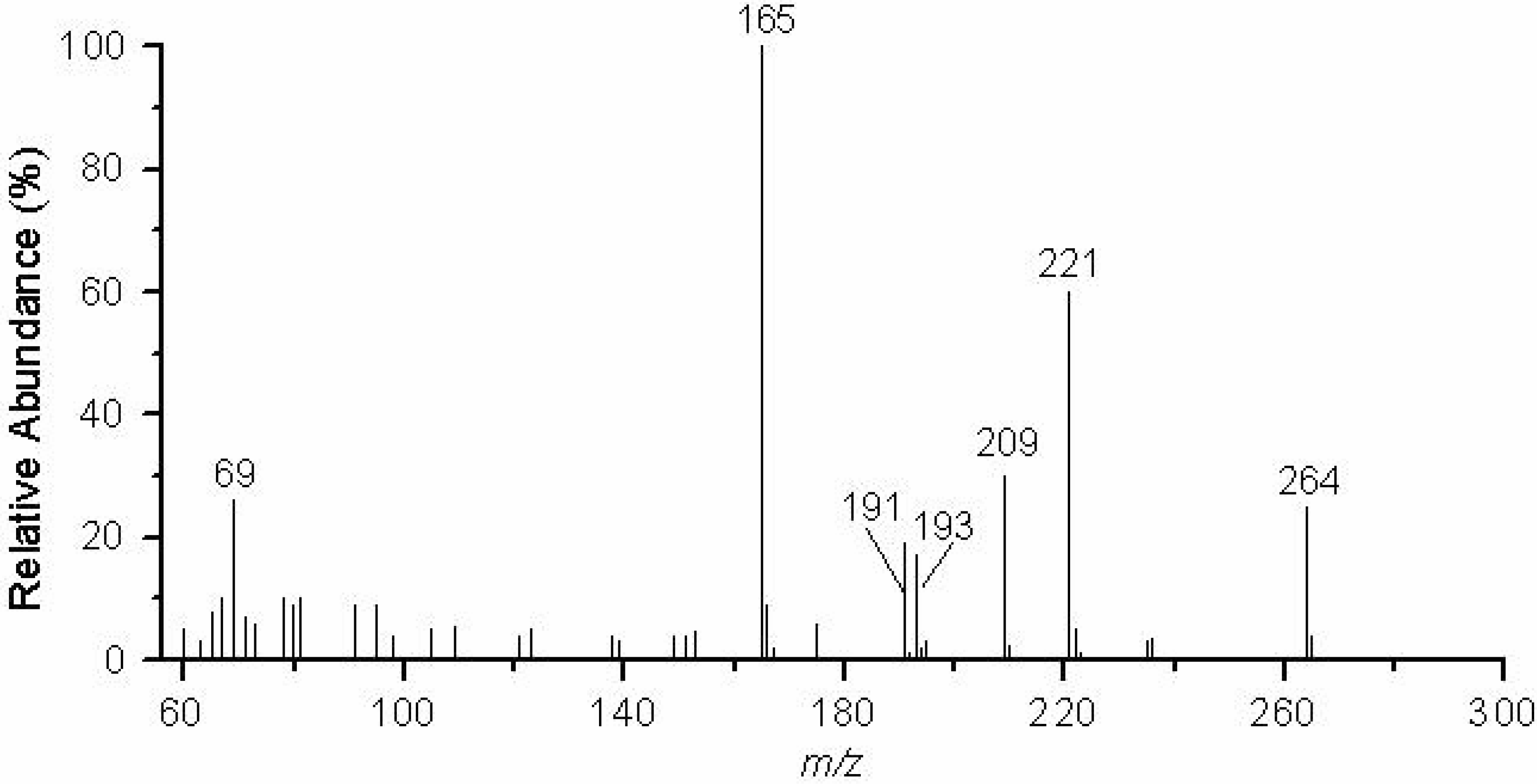
Figure 2.
EIMS (70 eV) of 5,7-dihydroxy-2,2-dimethyl-8-butyryl chroman (1).
Figure 2.
EIMS (70 eV) of 5,7-dihydroxy-2,2-dimethyl-8-butyryl chroman (1).
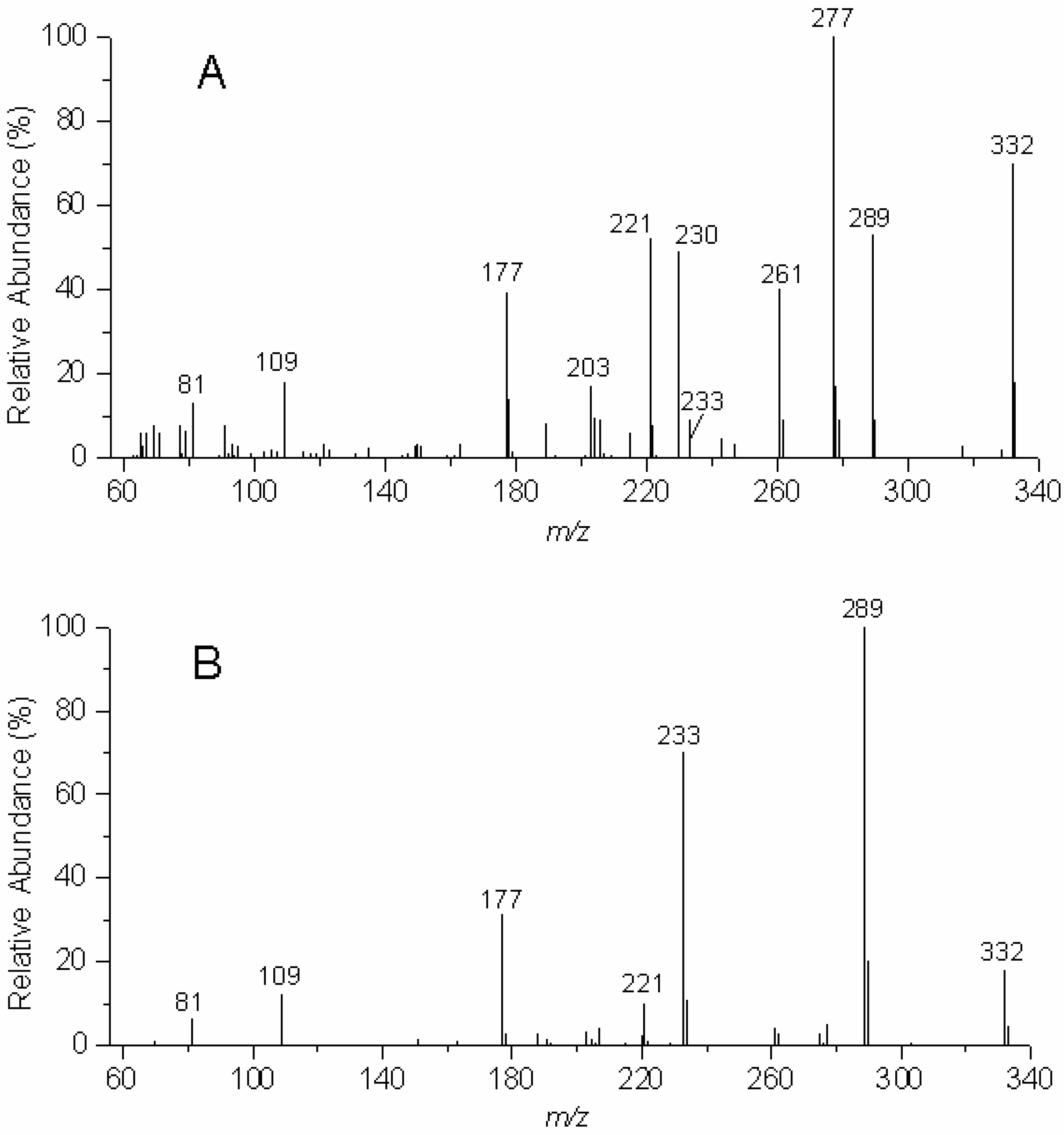
There are striking differences in the EIMS fragmentation patterns of the two isomeric benzodipyrans,
2 and
3 (see
Figure 3A and
Figure 3B and
Table 1). While loss of a fragment of 55 amu gives rise to the base peak fragment for
2, the cleavage β to the aromatic ring to render the characteristic Ar-C≡O:
+ fragment
15 at m/z 289 constitutes the base peak for
3. (See
Table 1). As discussed above, this fragment of 55 amu is typically observed in the EIMS of 2,2-dimethylchromans and accounts for the loss of the [CH
2=C(CH
3)CH
2] fragment in a retro Diels-Alder rearrangement and the proton transfer to the oxygen atom (
Scheme 1 and
Scheme 2) [
9,
10].
Figure 3.
EIMS (70 eV) of the "angular" benzodipyran 2 (A, upper panel) and the "linear" benzodipyran 3 (B, lower panel).
Figure 3.
EIMS (70 eV) of the "angular" benzodipyran 2 (A, upper panel) and the "linear" benzodipyran 3 (B, lower panel).
Table 1.
EIMS fragments (m/z) and their intensities (in %) for 2 and 3.
Table 1.
EIMS fragments (m/z) and their intensities (in %) for 2 and 3.
| m/z | 2 | 3 |
| 332 | 70 | 18 |
| 289 | 53 | 100 |
| 277 | 100 | 5 |
| 261 | 40 | 4 |
| 233 | 9 | 70 |
| 230 | 49 | - |
| 221 | 52 | 10 |
| 203 | 17 | 3 |
| 177 | 39 | 31 |
| 109 | 18 | 12 |
| 81 | 13 | 16 |
These pronounced differences in the fragmentation of the two isomers prompted us to subject structures
2 and
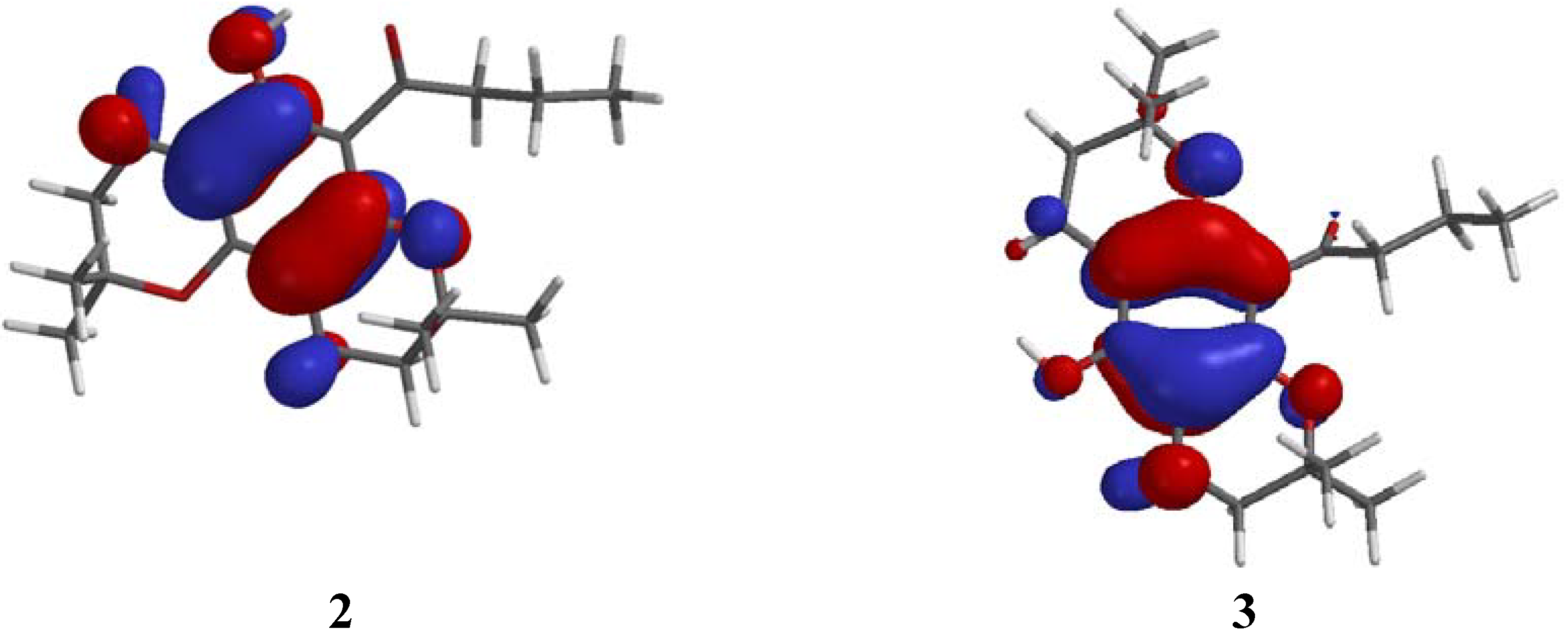
3 to semi-empirical calculations (AM1), in an attempt to compare the predicted sites of ionization with their associated fragmentation patterns. In both molecules, however, the highest occupied molecular orbital (HOMO) energy that is represented by the reciprocal value of the ionization potential (Koopmans' theorem [
15]) is located predominantly on the aromatic moiety (
Figure 4). In addition, the spin density maps (
Figure 5) for both radical cations
2.+ and
3.+, which are generated from AM1 calculations by removing one electron from the corresponding parent molecules
2 and
3, are located over the aromatic rings, in regions similar to those occupied by the HOMO energies of
2 and
3. Thus, neither the HOMO nor the spin density maps provided a suitable explanation for the differences in the fragmentation patterns of
2 and
3.
Figure 4.
Orbital representation of the HOMO energies of 2 and 3.
Figure 4.
Orbital representation of the HOMO energies of 2 and 3.
Figure 5.
Spin density maps of the radical cations 2.+ and 3.+.
Figure 5.
Spin density maps of the radical cations 2.+ and 3.+.
Table 2.
AM1 relative energies (kcal.mol-1) calculated for 2 and 3 and select cationic species derived from these benzodipyrans. The relative energies for 16 and its analogous ion from the "linear" isomer (not seen in EIMS) are also shown for comparative purposes.
Table 2.
AM1 relative energies (kcal.mol-1) calculated for 2 and 3 and select cationic species derived from these benzodipyrans. The relative energies for 16 and its analogous ion from the "linear" isomer (not seen in EIMS) are also shown for comparative purposes.
| | 2 | 3 | ΔHf2 - ΔHf3 |
Parent compounds
Radical cations
Ar-C≡O:+ | -175.701
1.568
25.401 | -170.990
6.618
18.654 | -4.711
-5.050
+6.747 |
![Molecules 09 00830 i001]()
**derived from 3 (not seen in EIMS) | |
19.062 | |
![Molecules 09 00830 i002]() |
12.856 | |
-6.206 |
Since the semi-empirical calculations did not offer an explanation for the diverse fragmentation patterns of
2 and
3, it was deemed appropriate to comparatively evaluate the relative energies (heat of formation ΔH
f) of the isomers
2 and
3, their radical cations
2.+ and
3.+, and the isomeric Ar-C≡O:
+ fragments
14 and
15 (see
Scheme 3 and
Scheme 4) that result from loss of the propyl group.
Because
2 and
3 are constitutional isomers their relative energies can be directly compared. The relative energies (
Table 2) reveal that the "angular" benzodipyran
2 is more stable than its "linear" isomer
3 by ca. 5 kcal.mol
-1. This energy advantage of
2 over
3 can easily be rationalized by the presence, in
2, of hydrogen bonding between the C=O group and the adjacent OH (d = 1.997 Å between O and H), resulting in a reduction of the entropy and an increased stabilization of the molecule. This hydrogen bonding was also illustrated by
1H NMR (
Figure 1). An exchangeable phenolic proton signal was observed at 4.95 ppm (s, 1H) in the spectrum of
3, but was absent in the spectrum of the "angular" isomer
2. In
2, this hydrogen bonding restricts the carbonyl group close to coplanarity (θ = 27°) with the aromatic ring while in
3, the carbonyl group opens to an angle of ca. 70° relative to the aromatic ring (see
Figure 4 and
Figure 5). Thus, both the parent benzodipyran
2 and its radical cation
2.+ are more stable than
3 and its corresponding radical cation
3.+. On the other hand, the Ar-C≡O:
+ fragment
14, derived from
2, is less stable than the Ar-C≡O:
+ fragment
15, derived from
3, by about 6.7 kcal.mol
-1. This indicates that loss of the propyl group from radical cations
2.+ and
3.+ is disfavored by nearly 12 kcal.mol
-1 for isomer
2 relative to isomer
3. Such an energy difference may account for partitioning to occur from radical cations
2.+ and
3.+ to yield fragments [Ar-C≡O:
+]
14 and
15 at
m/z 289 after loss of propyl or to the molecular ion at
m/z 277 from loss of the [C
4H
7] fragment (55 amu) from the benzodipyran moiety (see
Scheme 3 and
Scheme 4).
Scheme 3.
EI Induced fragmentation of the "angular" benzodipyran isomer 2.
Scheme 3.
EI Induced fragmentation of the "angular" benzodipyran isomer 2.
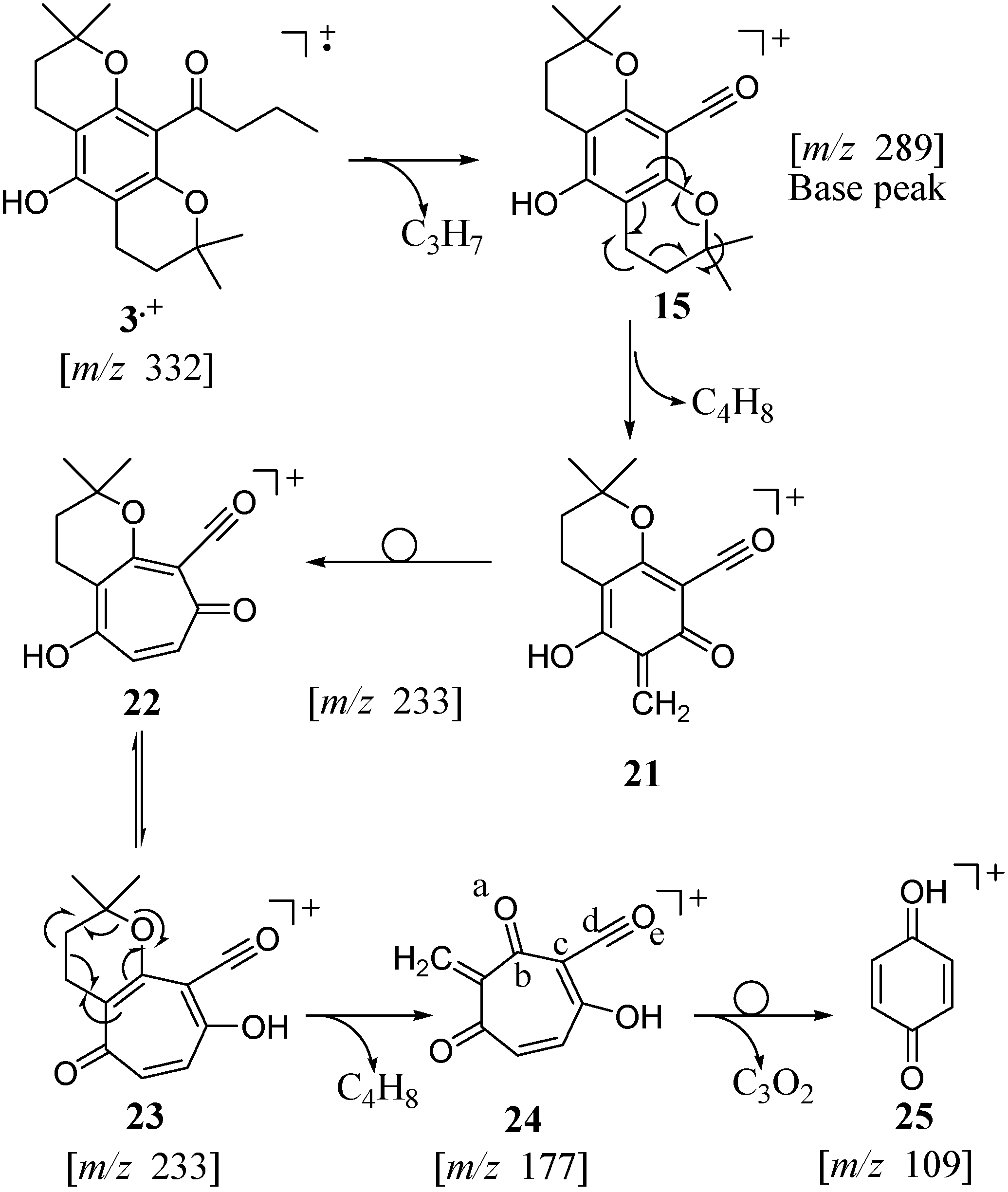
The fragmentation of
2 (
Scheme 3) proceeds by partitioning of the radical cation
2.+ to lose propyl by β cleavage relative to the aromatic ring rendering cation
14 at
m/z 289 and by loss of a fragment (55 amu) resulting from the fragmentation of one of the chroman moieties and the transfer of a proton to the oxygen atom yielding molecular ion
16 at
m/z 277. Further fragmentation of
14 successively leads to fragment ions at
m/z 233,
m/z 177 and
m/z 109 (the same fragmentation pathway is observed with
15 as shown in
Scheme 4 below). The fragment ion
16 most likely rearranges to the more stable hydroxytropylium ion
17 from which the second 2,2-dimethylpyran can fragment by loss of [CH
2=C(CH
3)
2] (56 amu) to yield the molecular ion
18 at
m/z 221. Fragment ion
19 at
m/z 261 most likely results from loss of the [CO-propyl] fragment (71 amu) directly from the parent ion
2.+ or sequentially from the fragment ion
14. Fragmentation of the chroman moiety would account for the molecular ion cluster
20 at
m/z 203–205 in the EIMS of
2 (see
Figure 3).
Scheme 4.
EI Induced fragmentation of the "linear" benzodipyran isomer 3.
Scheme 4.
EI Induced fragmentation of the "linear" benzodipyran isomer 3.
For the "linear" isomer
3, the loss of the propyl to yield
15 is followed by successive loss of the two [CH
2=C(CH
3)
2] fragments (56 amu) from the two chroman moieties affording molecular ions
21 at
m/z 233 and
24 at
m/z 177, respectively (
Scheme 4). In the fragmentation of
3, however, the retro Diels‑Alder rearrangements occur without proton transfer to the aromatic moiety. As previously discussed in the rearrangement of
1, and reported for
4 [
9], the quinone methide ion
21 presumably rearranges to a more stable tropylium ion
23. A molecular ion at
m/z 109 (
25, 12 % intensity) is likely to result from the loss of a [C
3O
2] fragment (68 amu). Although this C
3O
2 fragment was not confirmed, the loss of atoms a–e from
24 may afford the stable protonated paraquinone molecular ion
25.