Synthesis and Reactions of N-Methylbenzylammonium Fluorochromate(VI) on Silica Gel, a Selective and Efficient Heterogeneous Oxidant
Abstract
:Introduction
Results and Discussion
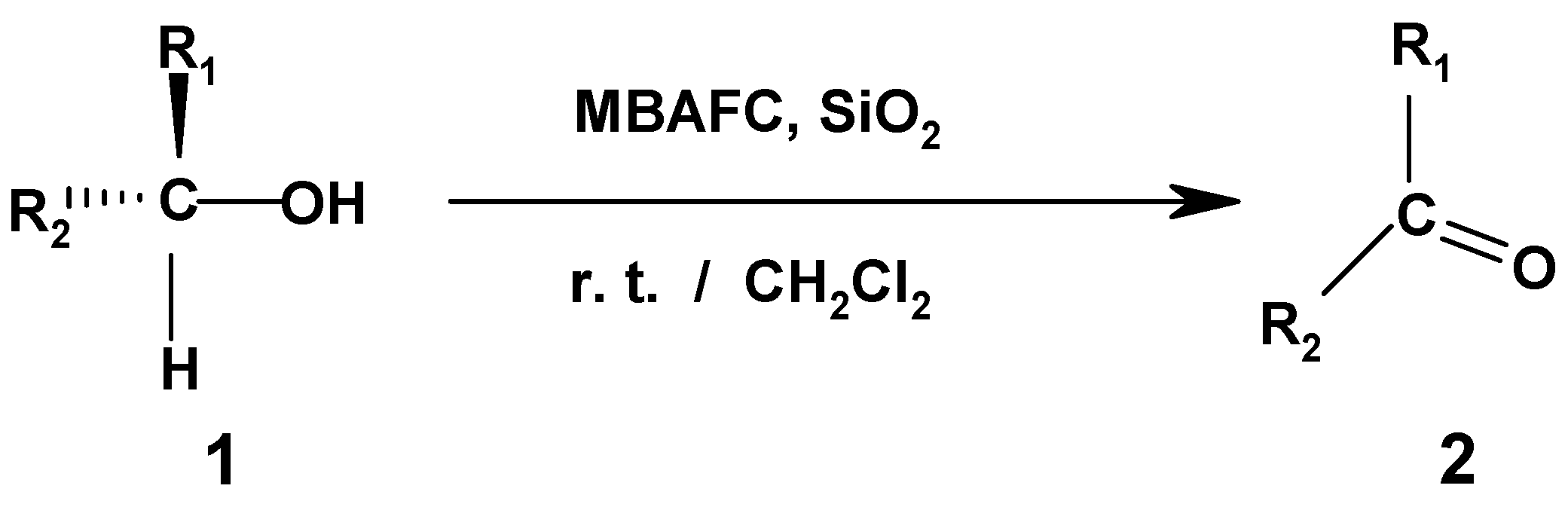
| Alcohol | Product c | MBAFC/Silicagel | MBAFC | PFC | IQFC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Molar Ratio ROH:OX | Time (min) | Yield c (%) | Time (min) | Yield (%) | Time (min) | Yield (%) | Time (min) | Yield (%) | ||
| Benzyl alcohol 1a | Benzaldehyde 2a | 1:1 (1:1.25)a | 10 | 93 | 60 | 85 | 45 | 90 | 60 | 91 |
| p-Chlorobenzyl alcohol, 1b | p-Chlorobenzaldehyde 2b | 1:1 | 15 | 91 | 75 | 89 | - | - | - | NR |
| p-Methoxybenzylalcohol, 1c | p-Methoxybenzaldehyde 2c | 1:1 (1:1.25)a | 17 | 93 | 80 | 80 | 50 | 90 | - | NR |
| p-Methylbenzylalcohol, 1d | p-Methylbenzaldehyde 2d | 1:1 | 20 | 92 | 70 | 89 | - | - | - | NR |
| p-Nitrobenzyl alcohol, 1e | p-Nitrobenzaldehyde 2e | 1:1 | 23 | 90 | 60 | 82 | - | - | - | NR |
| p-Bromobenzylalcohol, 1f | p-Bromobenzaldehyde 2f | 1:1 | 30 | 91 | 80 | 75 | - | - | - | NR |
| Cyclohexanol 1g | Cyclohexanone 2g | 1:1 (1:1.5)a | 24 | 92 | 75 | 65 | 210 | 89 | 240 | 90 |
| Cyclopentanol 1h | Cyclopentanone 2h | 1:1 | 19 | 87 | 95 | 70 | - | - | - | NR |
| 1-Phenylethanol 1l | Acetophenone 2l | 1:1 | 33 | 90 | 180 | 80 | - | - | - | NR |
| 3-Phenyl-2-propen-1-ol, 1m | 3-Phenylpropenal 2m | 1:1 (1:1.5)a | 26 | 89 | 150 | 80 | - | - | 300 | 70 |
| Benzoin 1n | Benzil 2n | 1:1 (1:1.25)a | 30 | 98 | 80 | 80 | 150 | 98 | 180 | 98 |
Conclusions
Experimental
General
Preparation of N-methylbenzylammonium fluorochromate(VI)
General Oxidation Procedure
Acknowledgements
References
- Collins, J.C.; W.W.; Franck, F.J. Dipyridine Chromium(VI) Oxide Oxidation of Alcohols in Dichloromethane. Tetrahedron Lett. 1968, 3363. [Google Scholar]
- Corey, E.J.; Fleet, G.W.J. Chromium Trioxide-3,5-Dimethylpyrazole Complex as a Reagent for Oxidation of Alcohols to Carbonyl Compounds. Tetrahedron Lett. 1973, 4499. [Google Scholar]
- Corey, E.J.; Suggs, J.W. Pyridinium Chlorochromate. An Efficient Reagent for Oxidatoin of Primary and Secondary Alcohols to Carbonyl Compounds. Tetrahedron Lett. 1975, 2647. [Google Scholar]
- Corey, E.J.; Schmidt, G. Useful Procedures for the Oxidation of Alcohols Involving Pyridinium Dichromate in Aprotic Media. Tetrahedron Lett. 1979, 399. [Google Scholar]
- Guziec, F.S.; Luzzio, F.A. The Oxidation of Alcohols Using 2,2'-Bipyridinium Chlorochromate. Synthesis 1980, 691. [Google Scholar]
- Bhattacharjee, M.N.; Chaudhuri, M.K.; Purkayastha, S. Some Aspects of Pyridinium Chlorochromate, C5H5NHCrO3F (PFC), Oxidations. Stoichiometry of Alcohols, Evidence for Oxygen Transfer, and Identity of the Reduced Chromium Species. Tetrahedron 1987, 43, 5389. [Google Scholar]
- Bhattacharjee, M.N.; Chaudhuri, M.K.; Dasgupta, H.S.; Roy, N. Pyridinium Fluorochromate; A New and Efficient Oxidant for Organic Substrates. Synthesis 1982, 588. [Google Scholar]
- Murugesan, V.; Pandurangan, A. Quinolinium Fluorochromate - A New Reagent for the Oxidation of Organic Compounds. Indian J. Chem. Sec. B 1992, 31B, 377. [Google Scholar]
- Srinivasan, R.; Ramesh, C.V.; Madhulatha, W.; Balasubramanian, K. Oxidation of Alcohols by Quinolinium Chlorochromate. Indian J. Chem. Sec. B. 1996, 35B, 480. [Google Scholar]
- Bora, U.; Chaudhuri, M. K. 3,5-Dimethylpyrazolium Fluorochromate(VI), C5H8N2H[CrO3F], (DmpzHFC): A Convenient New Reagent for Oxidation of Organic Substrates. Tetrahedron 2001, 57, 2445. [Google Scholar]
- Tajbakhsh, M.; Hosseinzadeh, R.; Yazdani-Niaki, M. Synthesis and Application of 2,6-Dicarboxypyridinium Chlorochromate as A New Oxidizing Reagent for Alcohols, Silyl ethers and THP ethers under Mild and Non-aqeous Conditions. J. Chem. Research (S) 2002, 2. [Google Scholar]
- Hosseinzadeh, R.; Tajbakhsh, M.; Yazdani-Niaki, M. 2,6-Dicarboxypyridinium Chloro-chromate: a Mild, Efficient and Selective Reagent for Oxidative Deprotection of Oximes to Carbonyl Compounds. Tetrahedron Lett. 2002, 43, 9413–9416. [Google Scholar]
- Tajbakhsh, M.; Heravi, M.M.; Mohanazadeh, F.; Sarabi, S.; Ghassemzadeh, M. N-Methyl-piperidinium Chlorochromate Adsorbed on Alumina: A New Deoximation Reagent. Monatsh. Chem. 2001, 132, 1229–1231. [Google Scholar]
- Kassaee, M.K.; Mahjob, A.R.; Ghammami, S. Tetramethylammonium fluorochromate(VI): a new and efficient oxidant for organic substrates. Tetrahedron Letters 2003, 44, 4555–45557. [Google Scholar]
- Kassaee, M.Z.; Sajjadi-Ghotbabadi, H.; Sayyed-Alangi, S.Z. N-Benzylmethylammonium Fluorochromate: A New Reagent for the Oxidation of Organic Compounds. The Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy, Pittcon 2003, March 9-14. Orlando, Florida, Abstracts; 2003; p. 1200-7P. [Google Scholar]
- Srinivasan, R.; Stanley, Preethi; Balasubramanian, K. Isoquinolinium Fluorochromate: A New and Efficient Oxidant for Organic Substrates. Synth. Commun. 1997, 27, 2057–2064. [Google Scholar]
- Sample availability: Available from the authors
© 2004 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kassaee, M.Z.; Sayyed-Alangi, S.Z.; Sajjadi-Ghotbabadi, H. Synthesis and Reactions of N-Methylbenzylammonium Fluorochromate(VI) on Silica Gel, a Selective and Efficient Heterogeneous Oxidant. Molecules 2004, 9, 825-829. https://doi.org/10.3390/91000825
Kassaee MZ, Sayyed-Alangi SZ, Sajjadi-Ghotbabadi H. Synthesis and Reactions of N-Methylbenzylammonium Fluorochromate(VI) on Silica Gel, a Selective and Efficient Heterogeneous Oxidant. Molecules. 2004; 9(10):825-829. https://doi.org/10.3390/91000825
Chicago/Turabian StyleKassaee, M. Z., S. Z. Sayyed-Alangi, and H. Sajjadi-Ghotbabadi. 2004. "Synthesis and Reactions of N-Methylbenzylammonium Fluorochromate(VI) on Silica Gel, a Selective and Efficient Heterogeneous Oxidant" Molecules 9, no. 10: 825-829. https://doi.org/10.3390/91000825




