Diazoamino Coupling of a 4-Sulfobenzenediazonium Salt with Some Cyclic Amines
Abstract
:Introduction
Results and Discussion
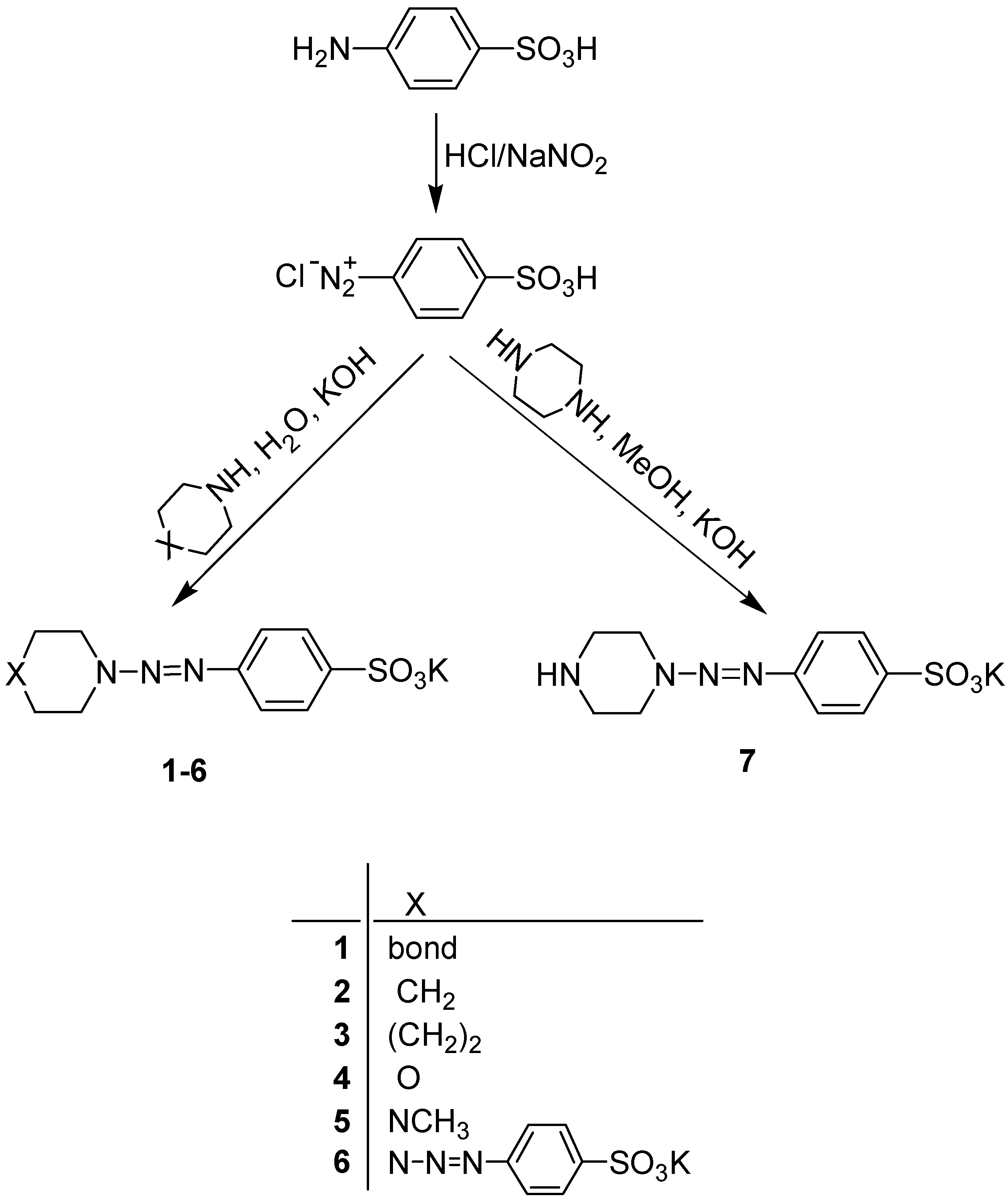
| Compound | Yield, % | M. p., oC | 1H-NMR, δ, ppm. | Raman spectra, Δν(N=N), cm-1 |
|---|---|---|---|---|
| 1 | 63 | 277-280 | 7.39 and 7.78 (4H, two d, J=9 Hz, C6H4), 3.60 (4H, br.s, CH2NCH2), 1,96 (4H, m, (CH2)2) | 1423 |
| 2 | 65 | 260-263 | 7.42 and 7.81 (4H, two d, J=9 Hz, C6H4), 3.66 (4H,br s, CH2NCH2), 1,58 (6H, br.s, (CH2)3) | 1433 |
| 3 | 60 | 267-270 | 7.42 and 7.77 (4H, two d, J=9 Hz, C6H4), 3.66 and 3.91 (4H, two t, CH2NCH2), 1.40-2.00 (8H, m, (CH2)4) | 1420 |
| 4 | 73 | 272-274 | 7.46 and 7.80 (4H, two dd, J=9 Hz, C6H4), 3.82 (4H, s, (CH2)O(CH2)) | 1436 |
| 5 | 58 | 264-267 | 7.47 and 7.79 (4H, two d, J=9 Hz, C6H4), 3.80 (4H, t, N-N(CH2)), 2.61 (4H, t, CH2NCH2), 2.30 (3H, s, CH3) | 1435 |
| 6 | 57 | 296-298 | 7.55 and 7.86 (4H, two d, J=9 Hz, C6H4), 4.08 (8H, s, CH2) | 1463 |
| 7 | 48 | 275-277 | 7.46 and 7.81 (4H, two d, J=9 Hz, C6H4), 3.74 (4H, t, =N-N(CH2)), 2.89 (4H, t, CH2NHCH2) | 1435IR ν(N-H), 3293cm-1 |
Conclusions
Experimental
General
References
- Lazny, R.; Poplawski, J.; Kobberling, J.; Enders, D.; Brase, S. Triazenes: a useful protecting strategy for sensitive secondary amines. Synlett 1999, 1304–1306. [Google Scholar] Wu, Z.; More, J.S. Iodine-promoted decomposition of 1-aryl-3,3-dialkyltriazenes: a mild method for the synthesis of aryl iodides. Tetrahedron Lett. 1994, 35, 5539–5542. [Google Scholar]
- Kolar, G.F. Synthesis of biologically active triazenes from isolable diazonium salts. Z. Naturforsch. 1972, 27, 1183–1185. [Google Scholar]
- Das, P.J.; Khound, S. Synthesis of 1-aryl-3,3-disubstituted triazenes on ion-exchange resin support. Tetrahedron 1994, 50, 9107–9113. [Google Scholar]
- Sava, G.; Giraldi, T.; Lassiani, L.; Nisi, C. Antimetastatic acction and hematological toxicity of p-(3,3-dimethyl-1-triazeno)benzoic acid potassium salt and 5-(3,3-dimethyl-1-triazeno)-imidazole-4-carboxamide used as prophylactic adjuvants to surgical tumor removal in mice bearing B16 melanoma. Cancer Res. 1984, 44, 64–68. [Google Scholar]
- Kažemėkaitė, M.; Šimkevičienė, V.; Talaikytė, Z. Antileucemic activity of 4-(3,3-dimethyltriazeno)-N-acylbenzenesulfonamides. Biologija (Vilnius) 2000, 242–244. [Google Scholar]
- Sava, G.; Perissin, L.; Lassiani, L.; Zabucchi, G. Antiinflammatory action of hydrosoluble dimethyltriazenes on the carrageenin-induced edema in Guinea pigs. Chem.-Biol. Interact. 1985, 53, 37–43. [Google Scholar]
- Kažemėkaitė, М.; Stumbrevičiūtė, Z.; Astrauskas, V. Aryltriazenes. 1. Synthesis and antiinflammatory activity of 4-(3,3-dimethyltriazene)-N-acylbenzenesulfamides. Khim. Pharm Zh. 1997, 31, 37–39. (in Russian). [Google Scholar]
- Giraldi, T.; Sava, G.; Cuman, R.; Nisi, C.; Lassiani, L. Selectivity of the antimetastatic and cytotoxic effects of 1-p-(3,3-dimethyl-1-triazeno)benzoic acid potassium salt, (±)-1,2-di(3,5-dioxopiperazin-1-il)propane and cyclophosphamide in mice bearing Lewis lung carcinoma. Cancer Res. 1981, 41, 2524–2528. [Google Scholar]
- Larsen, J.S.; Zahran, M.A.; Pedersen, E.B.; Nielsen, C. Synthesis of triazenopyrazole derivatives as potential inhibitors of HIV-1. Monatsh. Chem. 1999, 103, 1167–1173. [Google Scholar]
- Sava, G.; Geraldi, T.; Lassiani, L. Effects of isomeric aryldimethyltriazenes on Lewis lung carcinoma growth and metastases in mice. Chem.-Biol. Interact. 1983, 46, 131–136. [Google Scholar]
- Tietze, L.F.; Eicher, T. Reaktionen und Synthesen im organisch-chemishen Praktikum und Forschungslaboratorium. Thieme, G., Ed.; Stuttgart, New York, 1991. [Google Scholar]
- Zimmermann, F.; Lippert, Th.; Beyer, Ch.; Stebani, J.; Nuyken, O.; Wokaun, A. N=N vibrational frequencies and fragmentation patterns of substituted 1-aryl-3,3-dialkyl-triazenes: comparison with other high-nitrogen compounds. Applied Spectrosc. 1993, 47, 986–993. [Google Scholar]
- Scholz, A.; Wokaun, A. Assignment of N=N vibrational frequencies and tautomeric effects in the vibrational spectra of 1-aryl-3-alkyl-3-hydroxy-triazenes and their platinum complexes. Applied Spectrosc. 1995, 49, 1834–1840. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced oedema in hind paw of rat as the assay for antiinflammatory drugs. Proc. Soc. Exp. Biol.(N.Y.). 1962, 3, 544–547. [Google Scholar]
- Marek, J. Bentonite-induced rat paw oedema as a tool for simultaneous testing of prophylactic and therapeutic effects on anti-inflammatory and other drugs. Pharmazie 1981, 36, 46–49. [Google Scholar]
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kažemėkaitė, M.; Talaikytė, Z.; Niaura, G.; Butkus, E. Diazoamino Coupling of a 4-Sulfobenzenediazonium Salt with Some Cyclic Amines. Molecules 2002, 7, 706-711. https://doi.org/10.3390/70900706
Kažemėkaitė M, Talaikytė Z, Niaura G, Butkus E. Diazoamino Coupling of a 4-Sulfobenzenediazonium Salt with Some Cyclic Amines. Molecules. 2002; 7(9):706-711. https://doi.org/10.3390/70900706
Chicago/Turabian StyleKažemėkaitė, Marytė, Zita Talaikytė, Gediminas Niaura, and Eugenius Butkus. 2002. "Diazoamino Coupling of a 4-Sulfobenzenediazonium Salt with Some Cyclic Amines" Molecules 7, no. 9: 706-711. https://doi.org/10.3390/70900706




