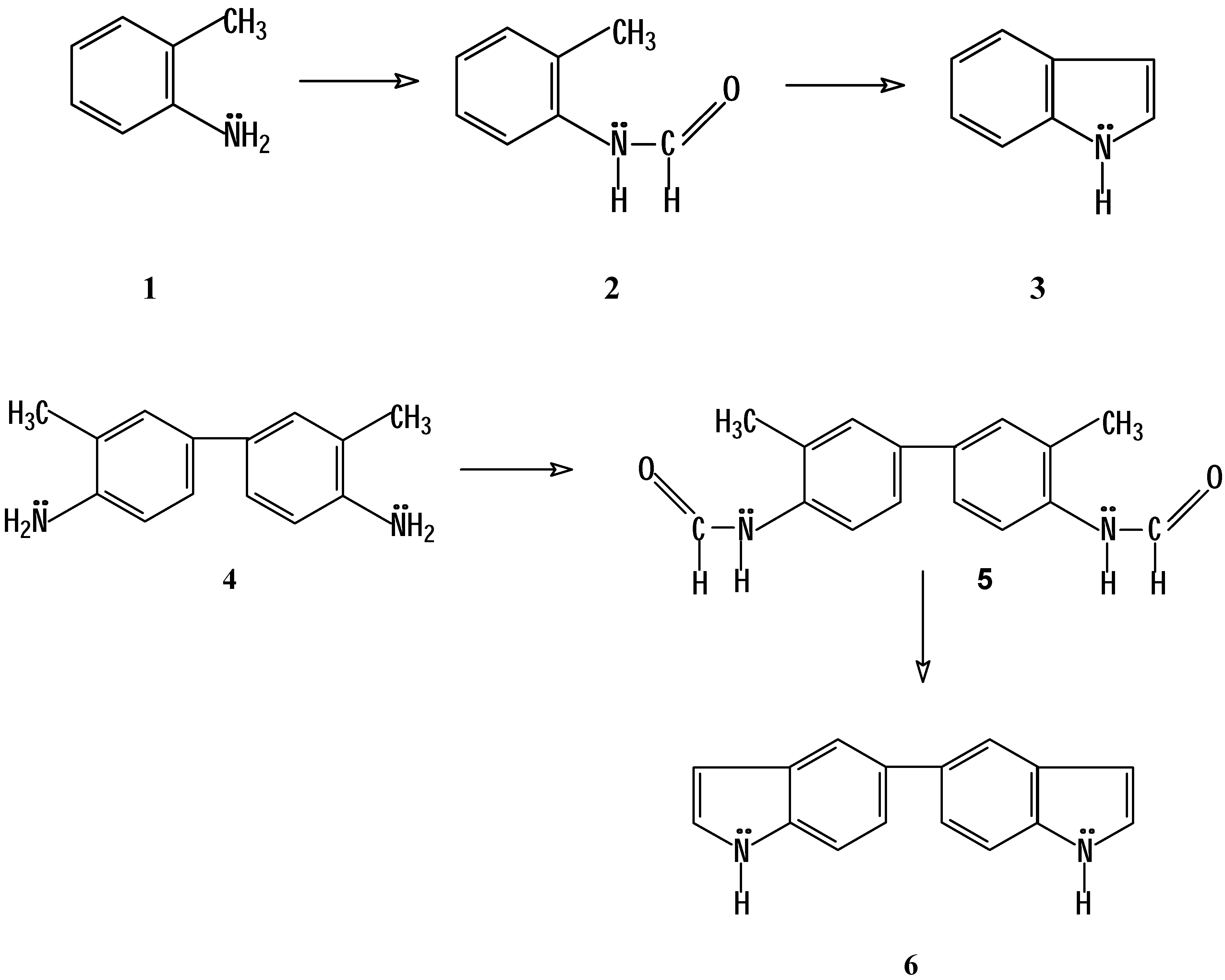
Introduction
The Madelung indole synthesis is a method for producing these heterocycles via a base-catalyzed thermal cyclization of
N-acyl-
o-toluidides [
1,
2]. At the same time, it is one of the few known reactions by which an unsubstitued indole nucleus can be obtained [
3,
4,
5]. Consequently, we decided to use this reaction in order to obtain 5,5´-biindole as a starting material for the synthesis of indoloquinolizines with potential antitumor activity [
6]. For our investigation we needed to synthesize
N-formyl-
o-toluidine (
2), starting from
o-toluidine (
1), as well as
N,N’-
bis-formyl-
o-tolidine (
5), starting from
o‑tolidine (
4), in order to transform them into indole (
3) and to 5,5´-biindole (
6), respectively (
Scheme 1).
During the spectroscopic characterization of the amides
2 and
5 we noted the presence of two isomers in solution (DMSO-
d6), observing double signals for the protons of the amine group and of the formyl group in the
1H-NMR spectra, as well as double signals for all carbons of both compounds in the
13C-NMR spectra. The ratio of the isomers, as deduced from the integration of the formyl proton signals, is approximately 3:1 in both compounds (
Figure 1 and
Figure 2). It is known that in a few cases single bond rotation is so slow that
cis and
trans isomers can be isolated even where no double bond exists [
7], e.g. in thioamides [
8,
9] and in certain amides [
10,
11,
12,
13,
14,
15,
16], because resonance gives the single bond some double bond character and slows rotation [
17].
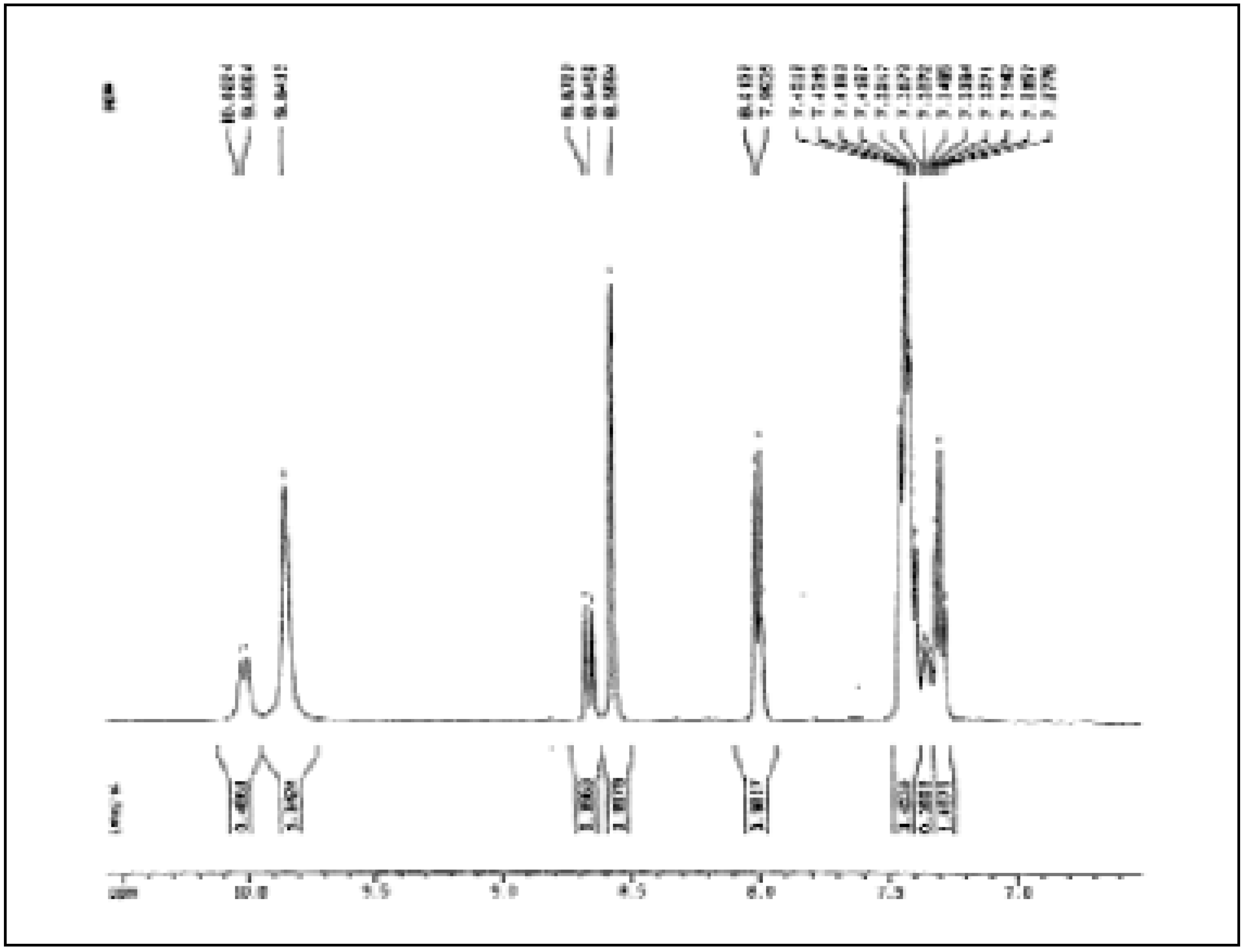
Figure 1.
Aromatic part of the 1H-NMR spectrum of 2 (in DMSO-d6 solution)
Figure 1.
Aromatic part of the 1H-NMR spectrum of 2 (in DMSO-d6 solution)
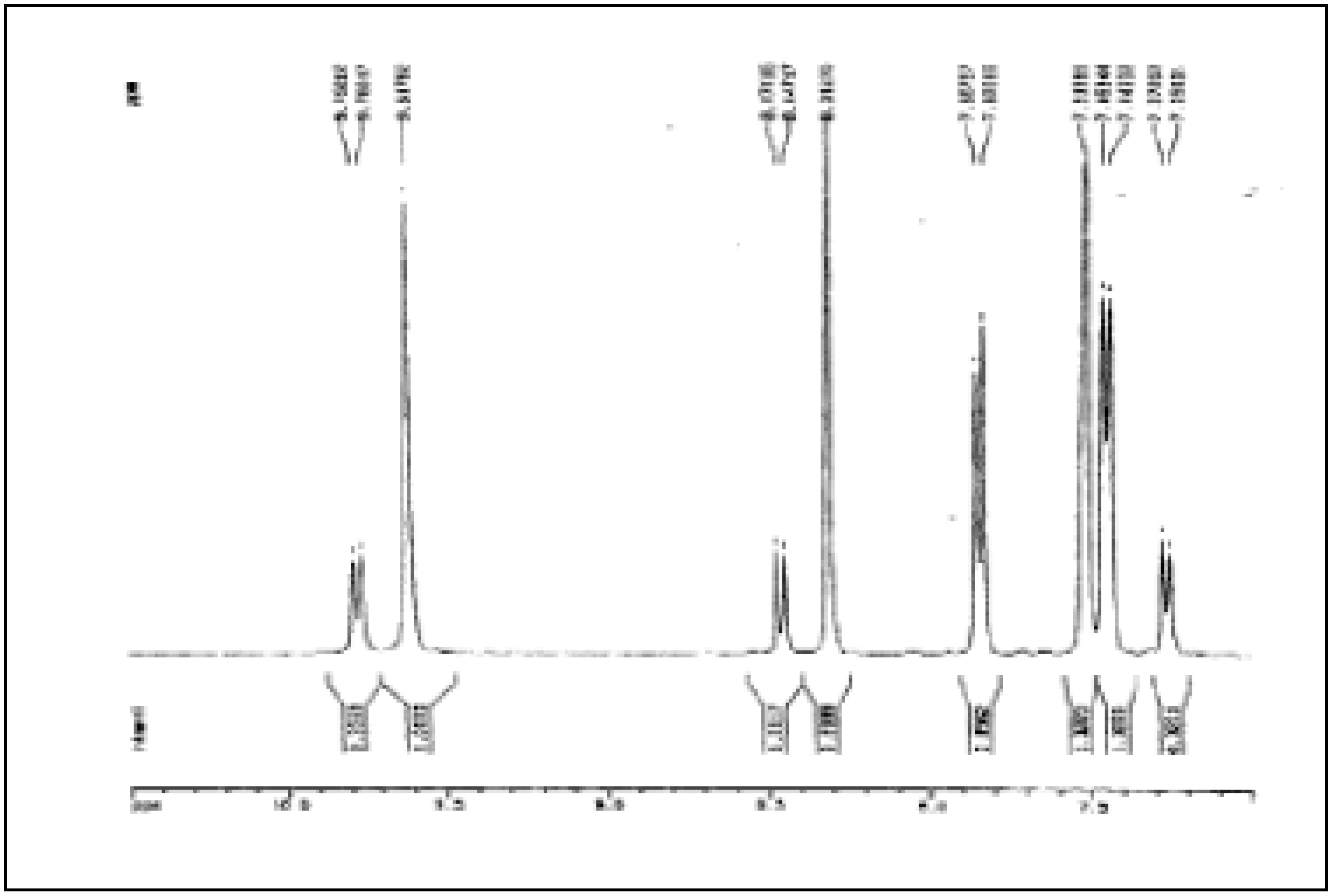
Figure 2.
Aromatic part of the 1H-NMR spectrum of 5 (in DMSO-d6 solution)
Figure 2.
Aromatic part of the 1H-NMR spectrum of 5 (in DMSO-d6 solution)
For example, in dimethylformamide (
7,
Scheme 2) the two methyl groups are non-equivalent due to the mentioned hindered rotation about the C-N bond. In the corresponding
1H-NMR spectrum two methyl signals are found at δ = 2.79 and 2.94, together with a singlet at δ = 8.0 for the formyl proton. If one saturates the methyl signal at δ = 2.94, the intensity of the formyl proton signal increases by 18 %. When instead the other methyl signal is saturated, a decrease of 2 % is observed [
18a]. In DMF the NOE experiments led to a correct assignment of the methyl signals. Since the signal of the isolated formyl proton is only enhanced when a particular one of the two methyl signals is saturated (the downfield one), this must correspond to the
cis methyl group (
trans to carbonyl group). Likewise, two signals for the methyl carbons are observed in the spectrum of
13C-NMR, at δ = 30 and 36, whereby the downfield signal corresponds to the methyl group,
trans to the carbonyl group [
19a].
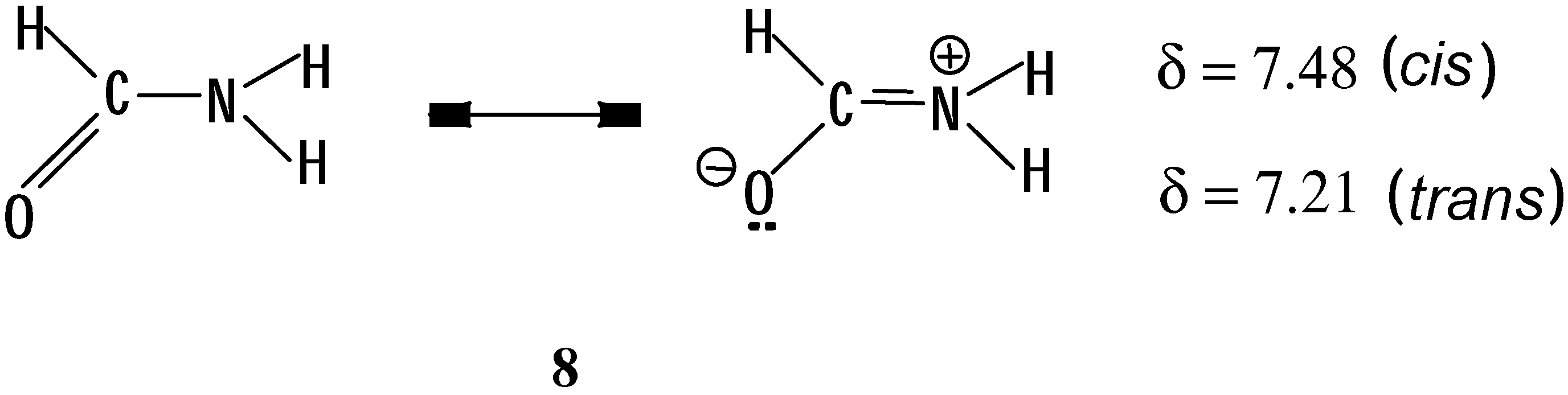
In the case of formamide (
8,
Scheme 3) the two protons on the nitrogen atom are also non
-equivalent [
19b]. Irradiating in the absorption frequency of
14N, 3 quartets centered at δ = 8.21, 7.48 and 7.21 are observed in the
1H-NMR spectrum [
20a,
21]. The downfield signal corresponds to the formyl proton, which presents two couplings of
J = 2.18 and 13.49 Hz. These couplings correspond to those we expected for the
cis N-H (δ = 7.48,
J = 2.18 and 2.60 Hz) with the formyl proton as well as for the
trans N-H (δ = 7.21,
J = 2.6 and 13.49 Hz) with the formyl proton. Thus, the two protons on the nitrogen atom show a geminal coupling constant
J = 2.6 Hz.
It is interesting to observe that the coupling constant of the
cis protons in formamide is of smaller magnitude (2.18 Hz) compared to that of the
trans protons (13.49 Hz), similar to what is seen in a typical double bond C=C [
18b].
Based on the reasoning described, it is expected that for monosubstituted formamides, as is the case of the amides 2 and 5, cis and trans isomers are present in solution which can be detected by way of NMR techniques. Thus, the purpose of our investigation was to assign in detail the configurations of the isomers observed in DMSO-d6 solutions of N-formyl-o-toluidine (2) and N,N’-bis-formyl-o-tolidine (5), by analyzing the spectra of 1H- and 13C-NMR, by running additional NOE experiments and using bidimensional spectra (COSY, HMQC, HMBC) for the full assignment of the signals.
Results and Discussion
The preferred conformation solution of
N-formyl-
o-toluidine (
2) in DMSO-
d6 is the
cis one, based on the fact that the
1H-NMR signals for the N-H and the formyl proton of the major isomer present appear as singlets at δ = 9.84 and 8.57 respectively, i.e. with a coupling constant
J≈ 0, whereas the signals corresponding to the minor isomer are present as doublets at δ = 10.01 and 8.66 respectively, showing a coupling constant
J ≈ 10.63 Hz, i.e. in the latter isomer a coupling constant of greater magnitude is observed, which is in accordance with the observation made for formamide (minor coupling constant for the
cis NH-CH protons and major coupling constant for the corresponding
trans protons) [
20a,
21].
The aforementioned observation is congruent with the one made in a series of
N-monoalkyl substituted formamides
9 (
Scheme 5) in which in their NMR spectra (mainly
13C) the
cis isomer predominates up to 80% over the
trans isomer [
20b].
Scheme 5.
R = methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, hexyl
In the case of the
N-methyl,
N-ethyl- and
N-tert-butylformamide signals for the formyl protons of the
cis isomer (d,
J = 0.3-2 Hz) as well as of
trans isomer (d,
J = 12 Hz) could be clearly observed in the
1H-NMR spectra (CDCl
3) which coincides again with the observation made for formamide. When analyzing the
13C-NMR spectra of these ten monoalkylamides it can be seen that the signals of the carbonyl carbon and those of the first two carbons of the alkyl substituent of the
trans isomer always appear downfield compared to the corresponding signals of the
cis isomer. Additional information about the configuration of the isomers of
2 (and also of
5) can be obtained with help of NOE experiments.
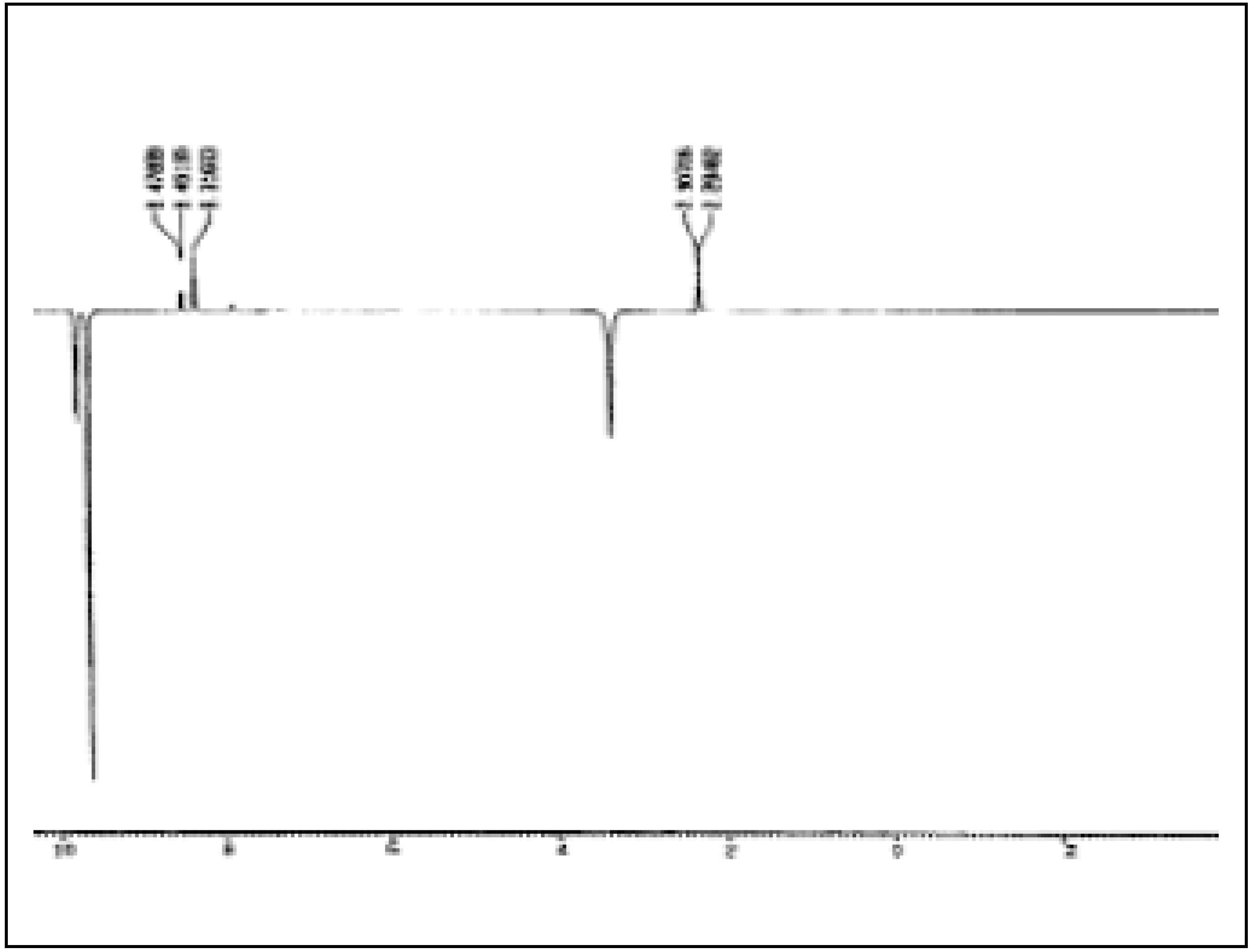
Figure 3 and
Figure 4 show the NOE experiments, in which the N-H signals of both isomers of
2 and
5 are saturated.
Figure 3.
NOE Experiment on 2.
Figure 3.
NOE Experiment on 2.
In the signals of the formyl protons we observe an increase by approx. 11% for the singlet at δ = 8.57 and by 8 % for the doublet at δ = 8.66, which indicates precisely that the signals of N-H and the formyl proton of the isomer in a greater porportion represent protons closer to each other and therefore correspond to the cis isomer. Additional analysis of the 1H-NMR spectra allows one to distinguish for cis-2 a doublet at δ = 8.0 (J = 7.88 Hz) for the proton in position 6 and a triplet at δ = 7.29 (J = 7.34 Hz) for the proton in position 4. The two remaining aromatic protons of cis-2 as well as all the aromatic protons of trans-2 appear as a multiplet in the 7.40 to 7.35 ppm region.
The signal of the aromatic protons of
2 are found at lower field compared to those observed for
o‑toluidine (δ = 6.59-7.01, CDCl
3, 400 MHz) [
20c]. This is due to the inductive effect (-I) of the amido group, which withdraws charge from the aromatic ring, in opposition to the amine group, which in the
o-toluidine introduces charge into the aromatic ring by means of resonance.
Figure 4.
NOE Experiment on 5.
Figure 4.
NOE Experiment on 5.
The effects of the substituents in the aromatic rings become normally apparent in the order
o>
p>
m and therefore it is expected that the proton in the
o-position to the amido group in
2 (position 6) is at lower field compared to the remaining aromatic protons. In fact, in
cis-2, this proton, 6-H, indeed appears at lower field compared to the others (δ = 8.00), however, it is strange that the corresponding proton in
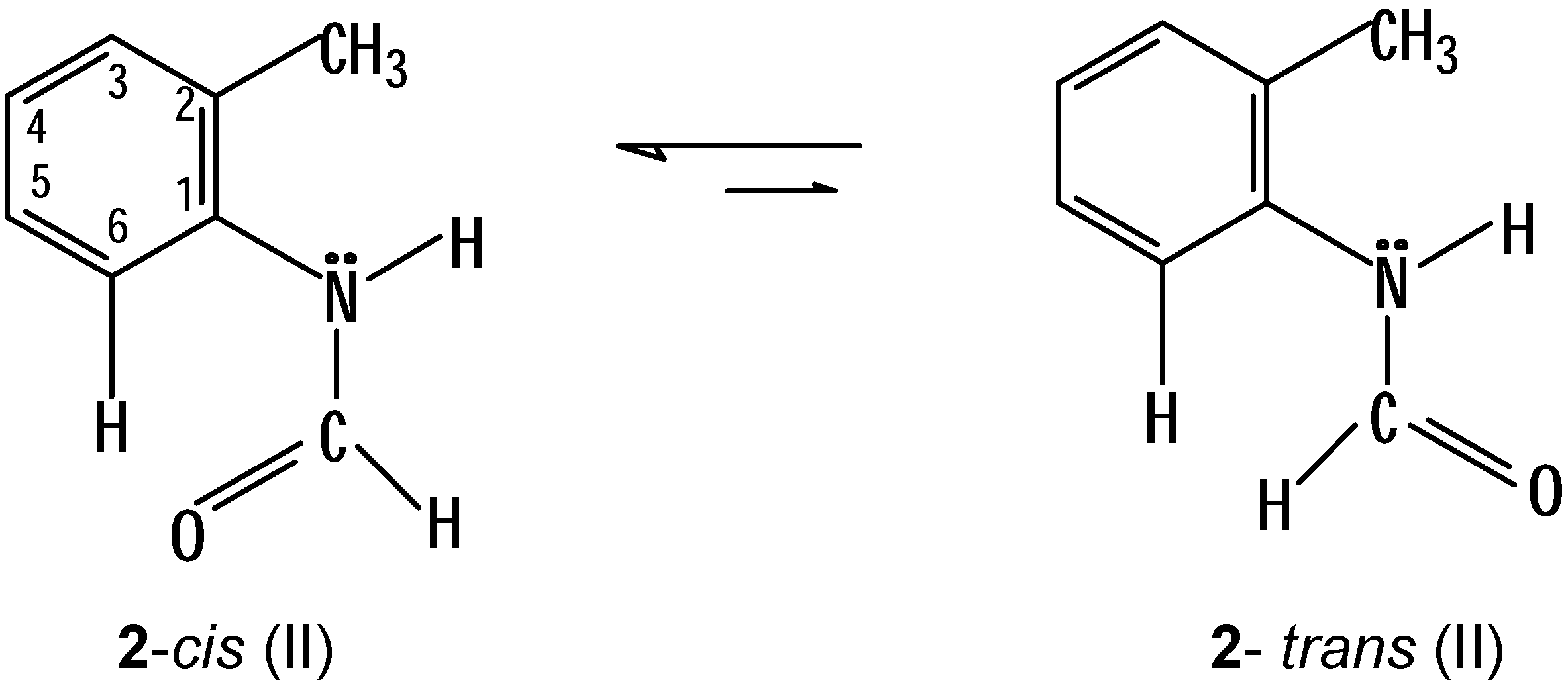
trans-2 is present at a higher field, in the multiplet at δ = 7.40-7.35. The foregoing observation can be explained, if we assume that the isomers of
2 are really present in the conformations (II) indicated below (
Scheme 6):
In these conformations (II) a steric hindrance between the formyl group and the methyl group of position 2 is avoided. However, a certain steric interaction between 6-H and the carbonyl group in cis-2 (and which is not present in trans-2) can be the cause for the additional downfield displacement of this proton.
The presence of the conformations II of
2 seems to be confirmed by the NOE experiment shown in
Figure 3. When irradiating in the signals of the N-H of the two isomers of
2 an increase in intensity of the signals of the methyl groups is observed, which confirms a spacial proximity of the protons under discussion. In the
13C-NMR spectrum it is possible to differentiate perfectly the signals of all carbons of both isomers of
2. In accordance with the expectations, the signals of the isomer in lower proportion (
trans-
2) are present at lower field compared to those corresponding to the isomer in higher proportion (
cis-
2).
The definite assignment of the chemical shifts of protons and carbons of both isomers of
2 can be achieved by taking into account the bidimensional experiments H,H-COSY, HMQC, HMBC as well as DEPT 135. These assignments are shown in
Table 1 and
Table 2.
Table 1.
1H(400 MHz) and 13C NMR (100MHz) Spectral Data for cis-2 in DMSO-d6
Table 1.
1H(400 MHz) and 13C NMR (100MHz) Spectral Data for cis-2 in DMSO-d6
| Position | δH , mult., J in Hz | δC | aDEPT 135 | COSY 1H-1H
correlations | bHMQC
1J CH | bHMBC (12Hz)
3J CH |
|---|
| H-C=O | 8.57, s | 160.18 | (+) CH | NH, 6-H | Formyl-H | -------- |
| NH | 9.84, s | NH | NH | Formyl-H | -------- | -------- |
| 1 | -------- | 135.73 | (0) Cq | -------- | -------- | Formyl-H
3-H
5-H |
| 2 | -------- | 129.60 | (0) Cq | -------- | -------- | 4-H
6-H |
| 2-CH3 | 2.47, s | 17.96 | (+) CH3 | 3-H | CH3 | |
| 3 | (7.45-7.33), m | 130.62 | (+) CH | 4-H | 3-H | 5-H |
| 4 | 7.30, t, 7.34 | 124.98 | (+) CH | 3-H | 4-H | 6-H |
| 5 | (7.45-7.33), m | 126.33 | (+) CH | 6-H | 5-H | 3-H |
| 6 | 8.00, d, 7.88 | 123.14 | (+) CH | 5-H, Formyl-H | 6-H | 4-H |
Table 2.
1H(400 MHz) and 13C-NMR (100MHz) Spectral Data for trans-2 in DMSO-d6
Table 2.
1H(400 MHz) and 13C-NMR (100MHz) Spectral Data for trans-2 in DMSO-d6
| Position | δH , mult., J in Hz | δC | aDEPT 135 | COSY 1H-1H
correlations |
|---|
| H-C=O | 8.66, d, 10.63 | 163.95 | (+) CH | NH |
| NH | 10.01, d, 10.63 | NH | NH | Formyl-H |
| 1 | -------- | 136.03 | (0) Cq | -------- |
| 2 | -------- | 129.60 | (0) Cq | -------- |
| 2-CH3 | 2.49, s | 17.82 | (+) CH3 | -------- |
| 3 | (7.45-7.33), m | 131.02 | (+) CH | -------- |
| 4 | (7.45-7.33), m | 125.63 | (+) CH | -------- |
| 5 | (7.45-7.33), m | 126.99 | (+) CH | -------- |
| 6 | (7.45-7.33), m | 122.09 | (+) CH | -------- |
In a similar way we found that in a solution of
N,N’-
bis-formyl-
o-tolidine in DMSO-
d6 the isomer with the
cis/cis conformation (
Scheme 7) predominates as NOE experiments are showing (see above,
Figure 4).
The assignment of the chemical shifts of protons and carbons of the two isomers of
5 are shown in
Table 3 and
Table 4 (due to the symmetry of
5, we refer only to positions 1 to 6, like
2).
Table 3.
1H(400 MHz) and 13C NMR (100MHz) Spectral Data for 5-cis in DMSO-d6
Table 3.
1H(400 MHz) and 13C NMR (100MHz) Spectral Data for 5-cis in DMSO-d6
| Position | δH , mult., J in Hz | δC | aDEPT 135 | COSY 1H-1H
correlations | bHMQC
1J CH | bHMBC (12Hz)
3J CH |
|---|
| H-C=O | 8.31, s | 159.84 | (+) CH | NH | Formyl-H | NH |
| NH | 9.62, s | NH | NH | Formyl-H | -------- | -------- |
| 1 | -------- | 134.87 | (0) Cq | -------- | -------- | Formyl-H
3-H, 5-H |
| 2 | -------- | 129.40 | (0) Cq | -------- | -------- | 6-H |
| 2-CH3 | 2.29, s | 17.93 | (+) CH3 | 3-H | CH3 | 3-H |
| 3 | 7.52, s | 128.22 | (+) CH | 2-CH3 | 3-H | 5-H |
| 4 | -------- | 135.69 | (0) Cq | -------- | -------- | 6-H |
| 5 | 7.45, d, 8.06 | 124.00 | (+) CH | 6-H | 5-H | 3-H |
| 6 | 7.85, d, 8.30 | 122.86 | (+) CH | 5-H | 6-H | NH |
Table 4.
1H(400 MHz) and 13C NMR (100MHz) Spectral Data for 5-trans in DMSO-d6
Table 4.
1H(400 MHz) and 13C NMR (100MHz) Spectral Data for 5-trans in DMSO-d6
| Position | δH , mult., J in Hz | δC | aDEPT 135 | COSY 1H-1H
correlations | bHMBC (12Hz)
3J CH |
|---|
| H-C=O | 8.46, d, 10.77 | 163.55 | (+) CH | NH | -------- |
| NH | 9.78, d, 10.77 | NH | NH | Formyl-H | -------- |
| 1 | -------- | 136.51 | (0) Cq | -------- | 5-H |
| 2 | -------- | 130.61 | (0) Cq | -------- | -------- |
| 2-CH3 | 2.29, s | 17.81 | (+) CH3 | -------- | -------- |
| 3 | 7.52, s | 128.68 | (+) CH | -------- | -------- |
| 4 | -------- | 134.96 | (0) Cq | -------- | -------- |
| 5 | 7.27, d, 8.08 | 124.56 | (+) CH | 6-H | -------- |
| 6 | 7.45, d, 8.06 | 122.03 | (+) CH | 5-H | -------- |