Synthesis of 5,6-Dihydropyridin-2(1H)-ones, 1,5,6,8,8a-Hexahydroisoquinolin-3(2H)-ones and 4a,5,6,7,8,8a-Hexahydroquinolin-2(1H)-ones by Intramolecular Wittig Reaction
Abstract
:Introduction
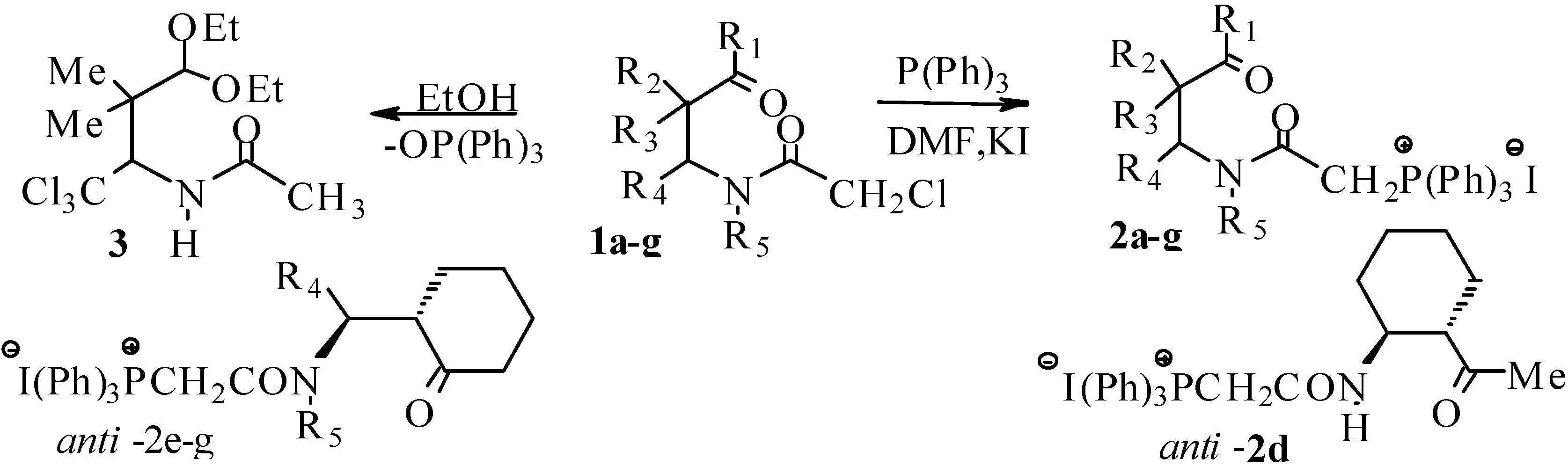
Results and Discussion

| Compound | 4a | 4b | 4c | trans-4d | trans-4e | trans-4f | trans-4g |
|---|---|---|---|---|---|---|---|
| m.p. °C | 135-61) | >210 decom | 147-81) | 176-71) | 183-41) | Oil | 172-31) |
| Compound | Chemical shifts δ (ppm) and coupling constants J (Hz) | ||||||
|---|---|---|---|---|---|---|---|
| =C-H (3J, 4J) | R1(3J, 4J) | R2(2J, 3J) | R3(2J, 3J) | R4 | NCH (3J, 4J) | R6 | |
| 4a | 5.79 m (1.5,1.5,1.5) | 1.93 m (1.5,1.0) | 2.58-2.40 m | 7.36 s | 4.72 m (9.0, 7.5, 0.6) | 5.70 s | |
| 4b | 5.53 d (3.8) | 7.30-7.01 m | 2.85 dd (16.1,7.0) | 2.67 dd (16.1,10.1) | 7.30-7.01 m | 3.93 m (10.1, 7.0, 3.8) | 8.10 m |
| 4c | 5.86 dd (10.1, 2.0) | 6.30 dd (10.1, 1.3) | 1.57 s | 1.43 s | – | 3.97 dd (4.6,1.3) | 7.37 s |
| trans-4d | 5.72 m (1.6, 1.6, 1.6) | 1.86 dd (1.6,1.6) | 2.17-1.02 m | 3.15 m (12.4, 10.8, 3.6) | 6.47 s | ||
| trans-4e | 5.76 dd (2.1, 2.1, 2.1) | 2.58-1.06 m | 7.37 s | 4.25 d (11,1) | 5.39 s | ||
| trans-4f | 5.82 dd (2.5, 2.5) | 2.53-0.60 m | 3.10-2.94 m | 7.30-7.15 m | 4.42 d (8.2) | 2.82 s | |
| trans-4g | 5.70 dd (1.4, 1.4, 1.4) | 2.47-1.23 m | 2.80-2.72 m | – | 3.82 dd (3.5,1.3) | 7.00 s | |
Conclusions
Experimental
General
Typical experimental procedure for the synthesis of salts 2a-g.
Typical experimental procedure for the syntheses of 4a-g.
Acknowledgments
References
- Fisyuk, A. S.; Bundel, Yu. G. 5,6-Dihydropyridin-2(1H)-ones and 5,6-dihydropyridine-2(1H)-thiones. Chem. Heterocycl. Compd. (N. Y.) 1995, 35, 125–145. [Google Scholar]
- Davis, D.A.; Gribble, G.W. A regioselective Diels-Alder synthesis of ellipticine. Tetrahedron Lett. 1990, 31, 1081–1084. [Google Scholar]
- Amat, M.; Llor, N.; Bosch, J.; Solans, X. Preparation of Enantiopure 6-(3-Indolyl)-2-piperidones and Conjugate Additions to a 3,4-Didegidro Derivative. Tetrahedron 1997, 53, 719–730. [Google Scholar]
- Heron, B.M. Heterocycles from Intramolecular Wittig, Horner and Wadsworth-Emmons Reactions. Heterocycles 1995, 41, 2357–2385. [Google Scholar]
- Marson, Ch.M.; Fallah, A. Catalytic C-amidoalkylations: synthesis of β-amido aldehydes by three-component condensations. Chem. Commun. 1998, 83–84. [Google Scholar]
- Lora-Tamayo, M.; Madroñero, R.; Leipprand, H. Beitrag zum chemischen Verhalten der 4H-1,3-Oxazine. Chem. Ber. 1964, 97, 2244–2250. [Google Scholar]
- Ikeda, K.; Terao, Y.; Sekiya, M. New Syntheses of α-N-Alkylacetamidomethylated Carbonyl Compounds. Chem. Pharm. Bull. 1981, 29, 1156–1159. [Google Scholar]
- Kober, R.; Papadopoulos, K.; Miltz, W.; Enders, D.; Steglich, W.; Reuter, H.; Puff, H. Synthesis of diastereo- and enantiomerically pure α-amino-γ-oxo acid esters by reaction of acyliminoacetates with enamines derived from 6-membered ketones. Tetrahedron 1985, 41, 1693–1701. [Google Scholar]
- Fisyuk, A.S.; Vorontsova, M.A.; Sagitullin, R.S. A New Synthesis of 3-Phenyl-5,6-Dihydropyridin-2(1H)-ones. Mendeleev Commun. 1993, 249–251. [Google Scholar]
- Kagoshima, H.; Hashimoto, Y.; Oguro, D.; Kutsuna, T.; Saigo, K. Triphenylphosphine/ Germanium (IV) Chloride Combination: A New Agent for the Reduction of α-Bromo Carboxylic Acid Derivatives. Tetrahedron Lett. 1998, 39, 1203–1206. [Google Scholar]
- Fisyuk, A. S.; Poendaev, N. V.; Bundel, Y.G. A New Route for Synthesis of 5,6-Dihydropyridine-2(1H)-ones, Pyridones-2 and (4-Hydroxy-2-oxopiperidyl-3)pyridinium chlorides by Intramolecular Cyclization of N-3-Oxoalkylchloroacetamides Derivatives. Mendeleev Commun. 1988, 12–14. [Google Scholar]
- Zaugg, H. E. α-Amidoalkylation at Carbon: Recent Advances – Part II. Synthesis 1984, 181–211. [Google Scholar]
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes.
Share and Cite
Fisyuk, A.S.; Poendaev, N.V. Synthesis of 5,6-Dihydropyridin-2(1H)-ones, 1,5,6,8,8a-Hexahydroisoquinolin-3(2H)-ones and 4a,5,6,7,8,8a-Hexahydroquinolin-2(1H)-ones by Intramolecular Wittig Reaction. Molecules 2002, 7, 124-128. https://doi.org/10.3390/70200124
Fisyuk AS, Poendaev NV. Synthesis of 5,6-Dihydropyridin-2(1H)-ones, 1,5,6,8,8a-Hexahydroisoquinolin-3(2H)-ones and 4a,5,6,7,8,8a-Hexahydroquinolin-2(1H)-ones by Intramolecular Wittig Reaction. Molecules. 2002; 7(2):124-128. https://doi.org/10.3390/70200124
Chicago/Turabian StyleFisyuk, Alexander S., and Nikolai V. Poendaev. 2002. "Synthesis of 5,6-Dihydropyridin-2(1H)-ones, 1,5,6,8,8a-Hexahydroisoquinolin-3(2H)-ones and 4a,5,6,7,8,8a-Hexahydroquinolin-2(1H)-ones by Intramolecular Wittig Reaction" Molecules 7, no. 2: 124-128. https://doi.org/10.3390/70200124




