An Improved Three Step Synthesis of (-)-3β-Hydroxycarvone from (-)-Carvone
Abstract
:Introduction
Results and Discussion
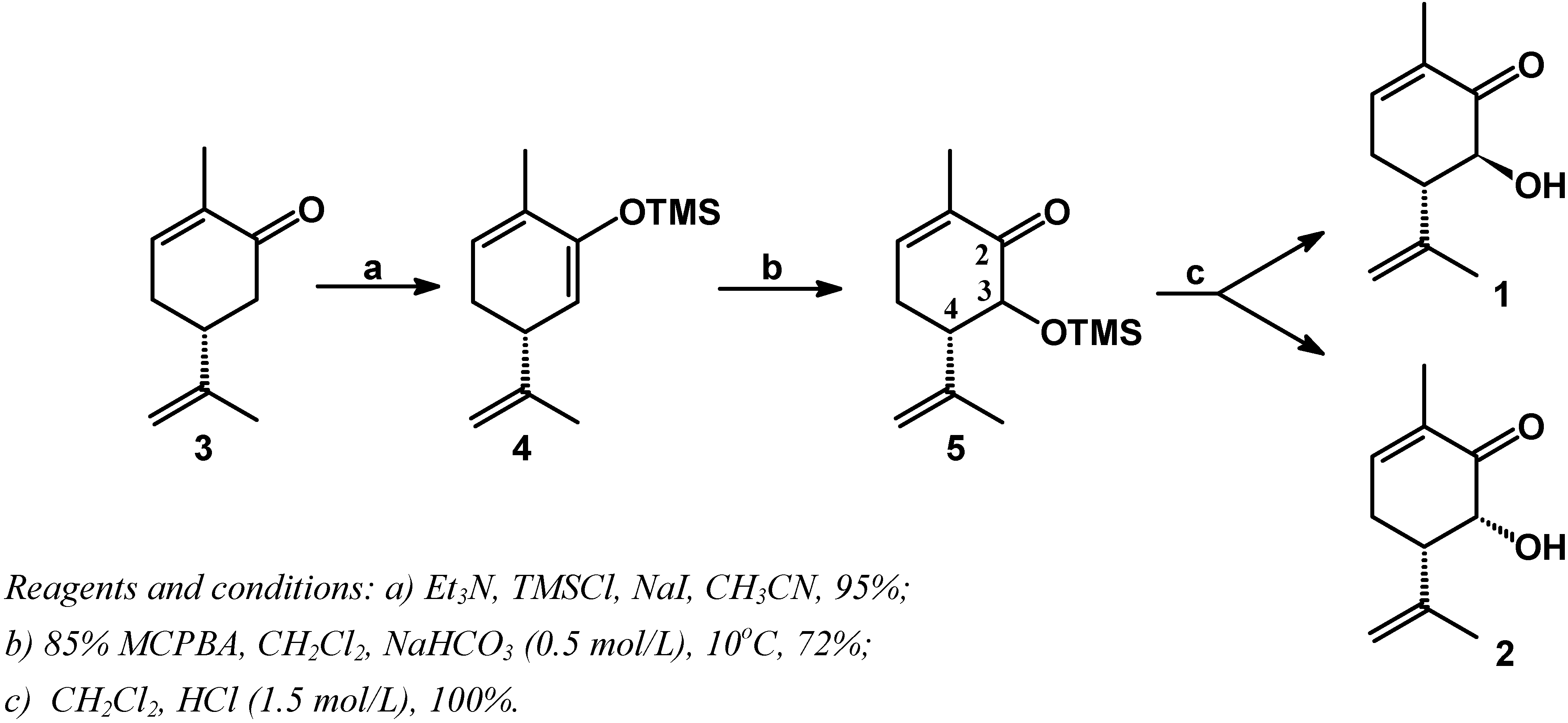
Conclusions
Experimental
General
(R)-3-(1-methylethenyl)-6-methyl-1,5-cyclohexadienyl trimethylsilyl ether (4).
(5S,6S)-(-)-6-hydroxy-2-methyl-5-(1-methylethenyl)-2-cyclohexen-1-one (1).
Acknowledgements
References
- Ho, T.-L. Enantioselective Synthesis: Natural Products from Chiral Terpenes; John Wiley & Sons: New York, 1995; pp. 123–183. [Google Scholar]
- Fraga, B. M. Nat. Prod. Rep. 1998, 15, 73–92.Fraga, B. M. Nat. Prod. Rep. 2000, 17, 483–504.Stadler, M.; Anke, H.; Sterner, O. Tetrahedron 1994, 50, 12649–12654.Bohlmann, F.; Zdero, C.; Scott, R. Phytochemistry 1987, 26, 1999–2006.Bohlmann, F.; Pathak, V. P.; Grenz, M.; Banerjee, S.; Wolfrum, C.; Barua, R. N.; Jakupovic, J. Phytochemistry 1987, 26, 1049–1052.
- Bohlmann, F.; Cardoso, J. M.; Jakupovic, J. Phytochemistry 1987, 26, 2321–2324.
- Hosokawa, T.; Nakahira, T.; Takano, M.; Murahashi, S-I. J. Mol. Catalysis 1992, 74, 489–498.
- Schulz, M.; Kluge, R.; Schuβler, M.; Hoffmann, G. Tetrahedron 1995, 51, 3175–3180.
- Hirai, Y.; Ito, K.; Nagaoka, H. Heterocycles 1998, 48, 235–238.
- Poirier, J. M.; Hennequin, L. Synth. Commun. 1985, 15, 217–244.
- Cazeau, P.; Duboudin, F.; Moulines, F.; Babot, O.; Dunogues, J. Tetrahedron 1987, 43, 2089–2100.Cazeau, P.; Duboudin, F.; Moulines, F.; Babot, O.; Dunogues, J. Tetrahedron 1987, 43, 2075–2087.
- Rubotton, G. M.; Gruber, J. M. J. Org. Chem. 1978, 43, 1599–1602.
- Rubotton, G. M.; Vazquez, M. A.; Pelegrina, D. R. Tetrahedron Lett. 1974, 4319–4322.
- Pennanen, S. I. Tetrahedron Lett. 1980, 21, 657–658.
- Hosokawa, T.; Inui, S.; Murahashi, S-I. Chem. Lett. 1983, 1081–1082.
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes.
Share and Cite
Dos Santos, R.B.; Brocksom, T.J.; Zanotto, P.R.; Brocksom, U. An Improved Three Step Synthesis of (-)-3β-Hydroxycarvone from (-)-Carvone. Molecules 2002, 7, 129-134. https://doi.org/10.3390/70200129
Dos Santos RB, Brocksom TJ, Zanotto PR, Brocksom U. An Improved Three Step Synthesis of (-)-3β-Hydroxycarvone from (-)-Carvone. Molecules. 2002; 7(2):129-134. https://doi.org/10.3390/70200129
Chicago/Turabian StyleDos Santos, Reginaldo B., Timothy J. Brocksom, Paulo R. Zanotto, and Ursula Brocksom. 2002. "An Improved Three Step Synthesis of (-)-3β-Hydroxycarvone from (-)-Carvone" Molecules 7, no. 2: 129-134. https://doi.org/10.3390/70200129



