Cyclization of N-(3-Oxoalkyl)chloroacetamides Under Basic Conditions. Synthesis of cis-3,4-Epoxypiperidin-2-ones
Abstract
:Introduction
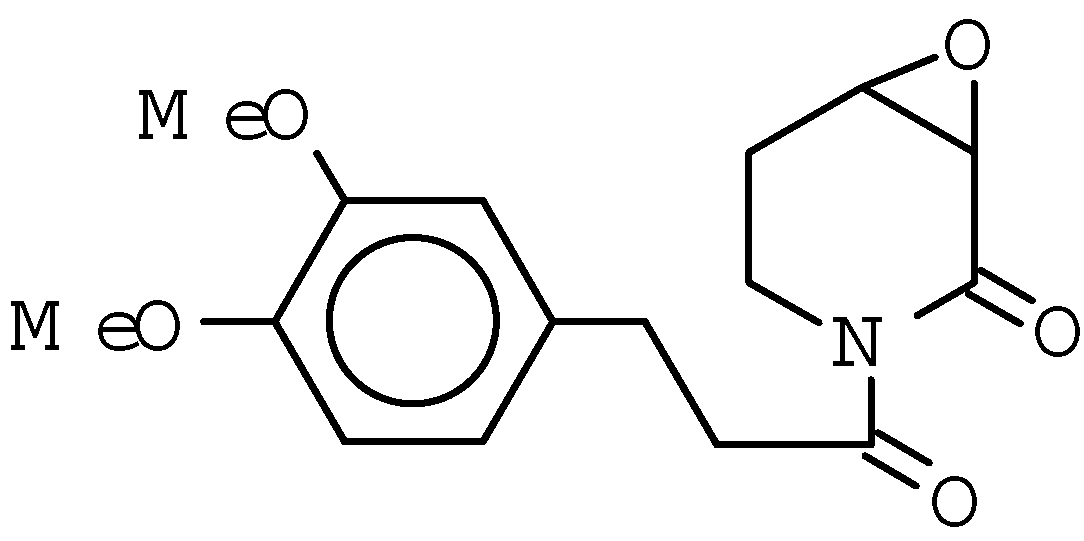
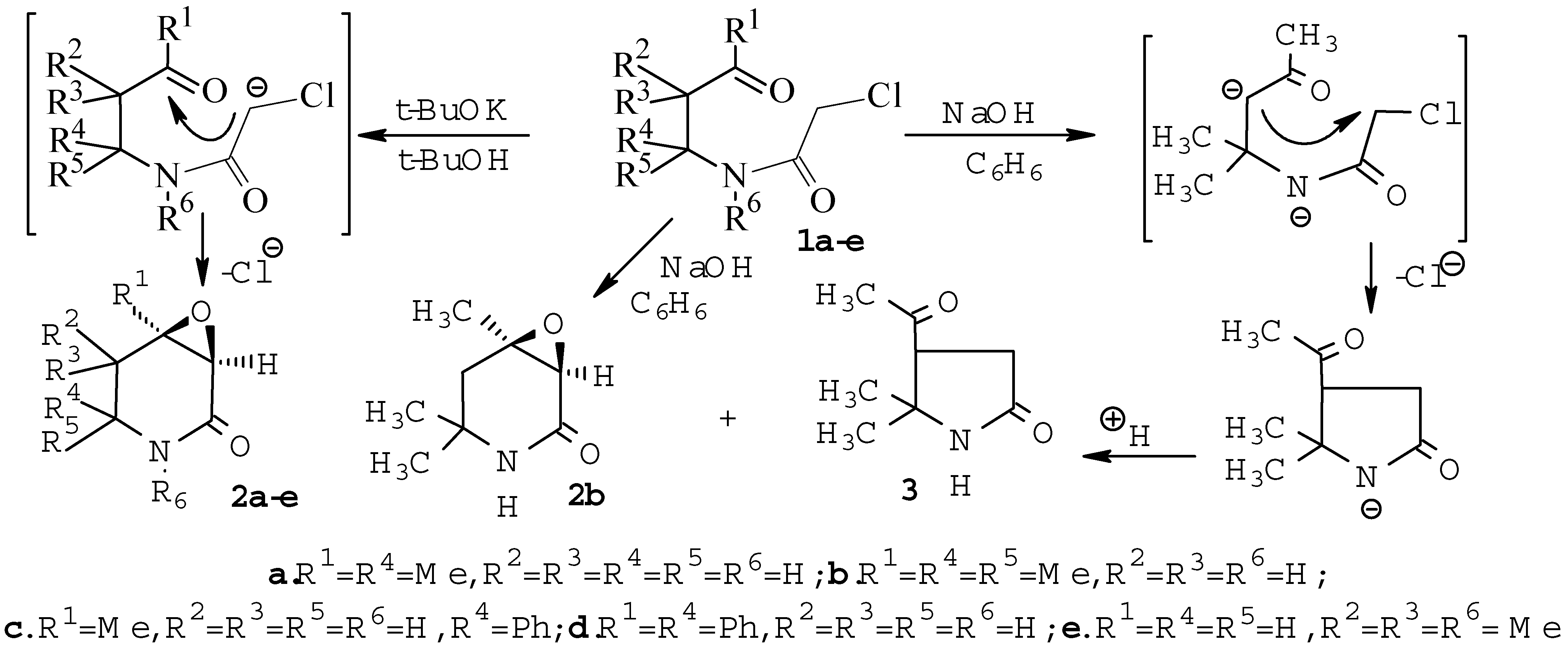
Results and Discussion
| Compound | 2a | 2b | 2c | 2d | 2e | 3b |
|---|---|---|---|---|---|---|
| Yield,% | 43 | 71 (28)1) | 27 | 10 | 59 | 241) |
| m.p., °C2) | 130-131 | 181-182 | >220(decomp) | >250(decomp) | 77-78 | 93-95 |
Conclusions
Experimental
General
Reaction of N-(3-oxoalkyl)chloroacetamides 1a-e with t-BuOK in t-BuOH-benzene media.
Reaction of 2-chloro-N-(1,1-dimethyl-3-oxobutyl)acetamide 1b with sodium hydroxide in benzene.
Spectral Data
Acknowledgments
References
- Capron, M. A.; Wiemer, D. F. Piplaroxide, an ant-repellent piperidine epoxide from Piper tuberculatum. J. Nat. Prod. 1996, 59, 794–795. [Google Scholar]
- Marson, Ch. M.; Grabowska, U.; Fallah, A.; Walsgrove, T.; Eggleston, D. S.; Baures, P. W. Stereoselective Syntheses of 5,6-Dihydro-2(1H)-pyridinones in Polyphospate Media. J.Org.Chem. 1994, 59, 291–296. [Google Scholar]
- Fisyuk, A. S.; Vorontsova, M. A.; Sagitullin, R. S. A New Synthesis of 3-Phenyl-5,6-Dihydropyridin-2(1H)-ones. Mendeleev Commun. 1993, 249–251. [Google Scholar]
- Fisyuk, A. S.; Vorontsova, M. A.; Ivanov, S. A. New synthesis of 3-aryl-5,6-dihydropyridin-2(1H)-ones. Khim. Geterotsikl. Soedin. 1994, 6, 8115–8128. [Google Scholar]
- Fisyuk, A. S.; Poendaev, N. V.; Bundel, Y. G. A. New Route for Synthesis of 5,6-Dihydropyridine-2(1H)-ones, Pyridones-2 and (4-Hydroxy-2-oxopiperidyl-3)pyridinium chlorides by Intramolecular Cyclization of N-3-Oxoalkylchloroacetamides Derivatives. Mendeleev Commun. 1998, 12–14. [Google Scholar]
- Lora-Tamayo, M.; Madroñero, R.; Leipprand, H. Beitrag zum chemischen Verhalten der 4H-1,3-Oxazine. Chem. Ber. 1964, 97, 2244–2250. [Google Scholar]
- Ikeda, K.; Terao, Y.; Sekiya, M. New Syntheses of α-N-Alkylacetamidomethylated Carbonyl Compounds. Chem. Pharm. Bull. 1981, 29, 1156–1159. [Google Scholar]
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes.
Share and Cite
Fisyuk, A.S.; Poendaev, N.V. Cyclization of N-(3-Oxoalkyl)chloroacetamides Under Basic Conditions. Synthesis of cis-3,4-Epoxypiperidin-2-ones. Molecules 2002, 7, 119-123. https://doi.org/10.3390/70200119
Fisyuk AS, Poendaev NV. Cyclization of N-(3-Oxoalkyl)chloroacetamides Under Basic Conditions. Synthesis of cis-3,4-Epoxypiperidin-2-ones. Molecules. 2002; 7(2):119-123. https://doi.org/10.3390/70200119
Chicago/Turabian StyleFisyuk, Alexander S., and Nikolai V. Poendaev. 2002. "Cyclization of N-(3-Oxoalkyl)chloroacetamides Under Basic Conditions. Synthesis of cis-3,4-Epoxypiperidin-2-ones" Molecules 7, no. 2: 119-123. https://doi.org/10.3390/70200119




