Crystal and molecular structure of cis-Dichlorobis(triphenylphosphite) Platinum(II)
Abstract
:Introduction
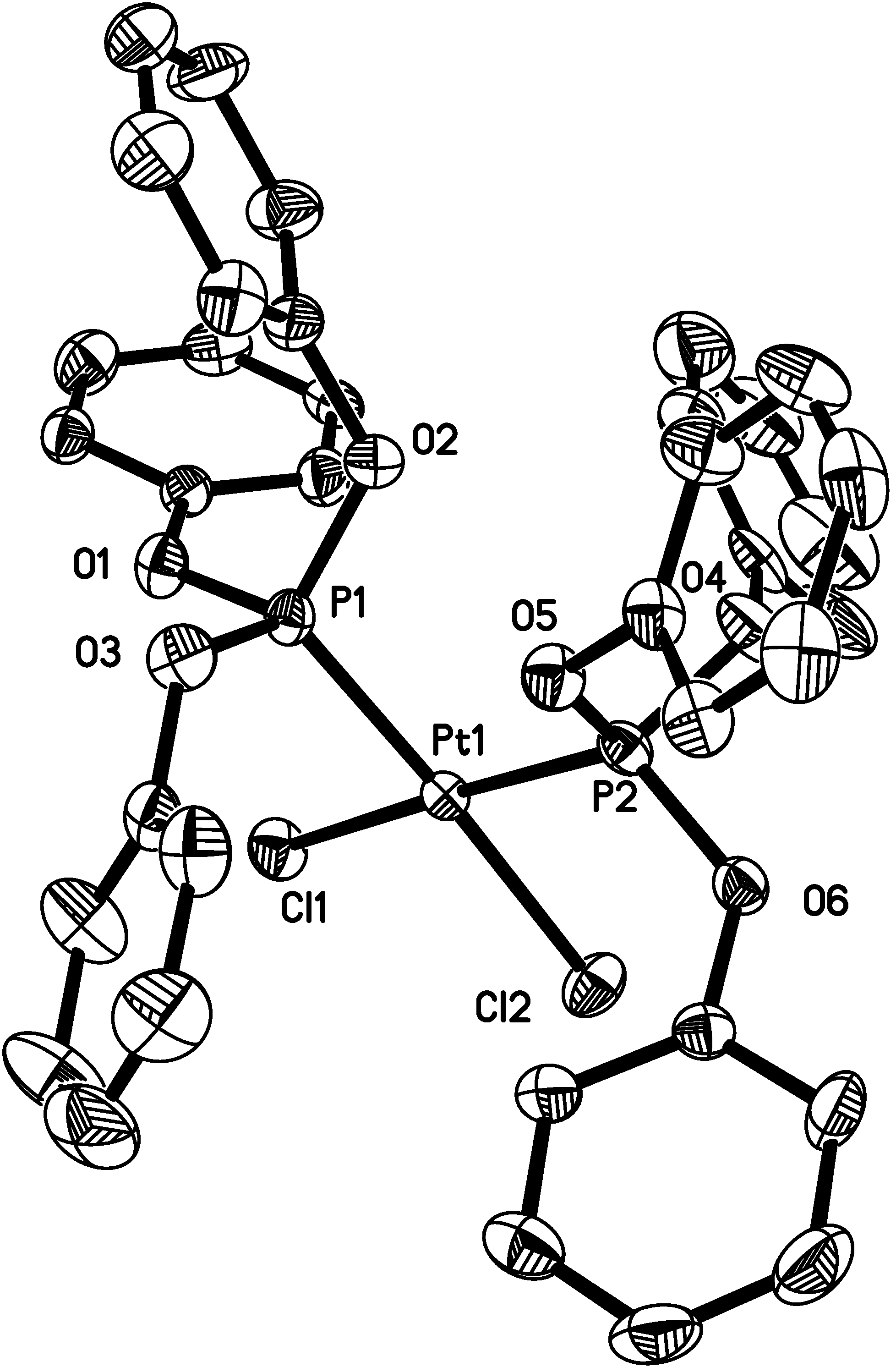
Results and discussion
| Formula weight | 886.59 |
| Temperature | 213(2) K |
| Wavelength | 0.71073 A |
| Crystal system, space group | orthorhombic, P 21 21 21 |
| Unit cell dimensions | a = 10.4315 (13 ) Ǻ alpha = 90 deg. |
| b = 14.0635 (16) Ǻ beta = 90 deg | |
| c = 23.505 ( 3 ) Ǻ gamma = 90 deg. | |
| Volume | 3448.3 ( 7 ) Ǻ 3 |
| Z, calculated density | 4, 1.708 Mg/m3 |
| Absorption coefficient | 4.164 mm-1 |
| F (000) | 1744 |
| Crystal size | 0.68 × 0.20 × 0.16 mm |
| Theta range for data collection | 1.73 to 24.26 deg. |
| Index ranges | -11<=h<11, -16<=k<=16, -26<=1<=26 |
| Reflections collected / unique | 22003/5449 [R(int)=0.0534] |
| Completeness to 2theta = 24.26 | 98.0% |
| Max. and min. transmission | 0.5554 and 0.1640 |
| Refinement method | full-matrix least-squares on F2 |
| Data / restraints / parameters | 5449 / 0 /424 |
| Goodness-of-fit on F∩2 | 0.943 |
| Final r indices [I>2sigma(I)] | R1 = 0.0203, WR2 = 0.0410 |
| R indices (all data) | R1 = 0.0280, WR2 = 0.0425 |
| Absolute structure parameter | -0.018(5) |
| Largest diff. peak and hole | 0.559 and -0.783 e.A−3 |
| Absorption correction | numerical (X-SHAPE : Stoe, 1997) |
| Tmin =0.1640, Tmax =0.5554 | |
| 22003 measured reflections | |
| 5449 independent reflections | |
| 4677 reflections with> 2sigma(I |
| Distances (Ǻ) | |||
| O1-P1 | 1.579(3) | O6-P2 | 1.577(3) |
| Angles (°) | |||
| O1-P1-O2 | 104.69(17) | O4-P2-Pt1 | 117.27(14) |
| X | Y | Z | U(eq) | |
| C(1) | -2590(4) | 790(3) | 676(2) | 23(1) |
Experimental
Acknowledgements
References
- Kitano, Y.; Ashida, T. Acta Crystallogr. 1983, C39, 1015.
- Davies, J.A.; Pinkerton, A.A.; Staples, R.J. Acta Crystallogr. 1990, C48, 48.
- Sabounchei, S.J.; Naghipour, A; Bickley, J.F. Acta Crystallgr. 2000, C56, 280.
- Allen, F.A.; Pidcok, A.; Waterhouse, C.R. J. Chem. Soc. (A) 1970, 2087.
- Ahmed, N.; Ainscough, E.W.; James, T.A.; Robinson, S.D. J. Chem. Soc. Dalton Trans. 1973, 1148.
- EXPOS, Stoe IPDS Software for Publication, version 2.79. Stoe IPDS: Darmstadt, Germany, Stoe 1997a.
- Cell Program for Cell Refinement; Version 2.79. Stoe IPDS: Darmstadt, Germany, Stoe 1997b.
- INTEGRATE Program for Reduction of IPDS Data, Version 2.79. Stoe IPDS: Darmstadt, Germany, Stoe 1997c.
- Sheldrick, G.M. Acta Crytallogr. 1990, A46, 467.
- Sheldrick, G.M. SHELXL 97 release 97-1; Program for Refinement of [3d] Crystal Structure; University of Göttingen: Germany, 1997.
- Sample Availability: The product reported in this paper is available from MDPI
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Sabounchei, S.J.; Naghipour, A. Crystal and molecular structure of cis-Dichlorobis(triphenylphosphite) Platinum(II). Molecules 2001, 6, 777-783. https://doi.org/10.3390/60900777
Sabounchei SJ, Naghipour A. Crystal and molecular structure of cis-Dichlorobis(triphenylphosphite) Platinum(II). Molecules. 2001; 6(9):777-783. https://doi.org/10.3390/60900777
Chicago/Turabian StyleSabounchei, Seyyed Javad, and Ali Naghipour. 2001. "Crystal and molecular structure of cis-Dichlorobis(triphenylphosphite) Platinum(II)" Molecules 6, no. 9: 777-783. https://doi.org/10.3390/60900777




