General
Melting points were measured on a Koffler hot stage apparatus and are uncorrected. Purity of compounds was confirmed by elemental analysis on a Perkin-Elmer, model 2400 analyzer. The reaction course was monitored by TLC on Silufol (Kavalier) and Alumina 60 F
254 neutral (Merck) TLC plates. Preparative column chromatography was performed on Kavalier 40/100 μm silica gel and Merck Kieselgel 60 F25. The infrared absorption spectra of compounds
1, and
5-7 were measured in CHCl
3 on an IR75 (Zeiss Jena) spectrometer in the region 400-4000 cm
-1. The
1H-NMR spectrum of
5 was measured on a TESLA BS 487 A (80 MHz),
1H- and
13C-NMR spectra of compounds
1, 6, 7 on a Varian Gemini 2000 (300 MHz) in deuterochloroform, using tetramethylsilane as an internal standard. The electron impact mass spectra of
7 were recorded on a Finnigan SSQ 700 spectrometer at an ionization energy of 70 eV. Methyl L-cysteinate hydrochloride, pyridinium chlorochromate (PCC), p-chloranil and 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) from Fluka, Merck and Avocado were used as obtained without further purification. 1-(tert-Butoxycarbonyl)indole-3-carboxaldehyde (
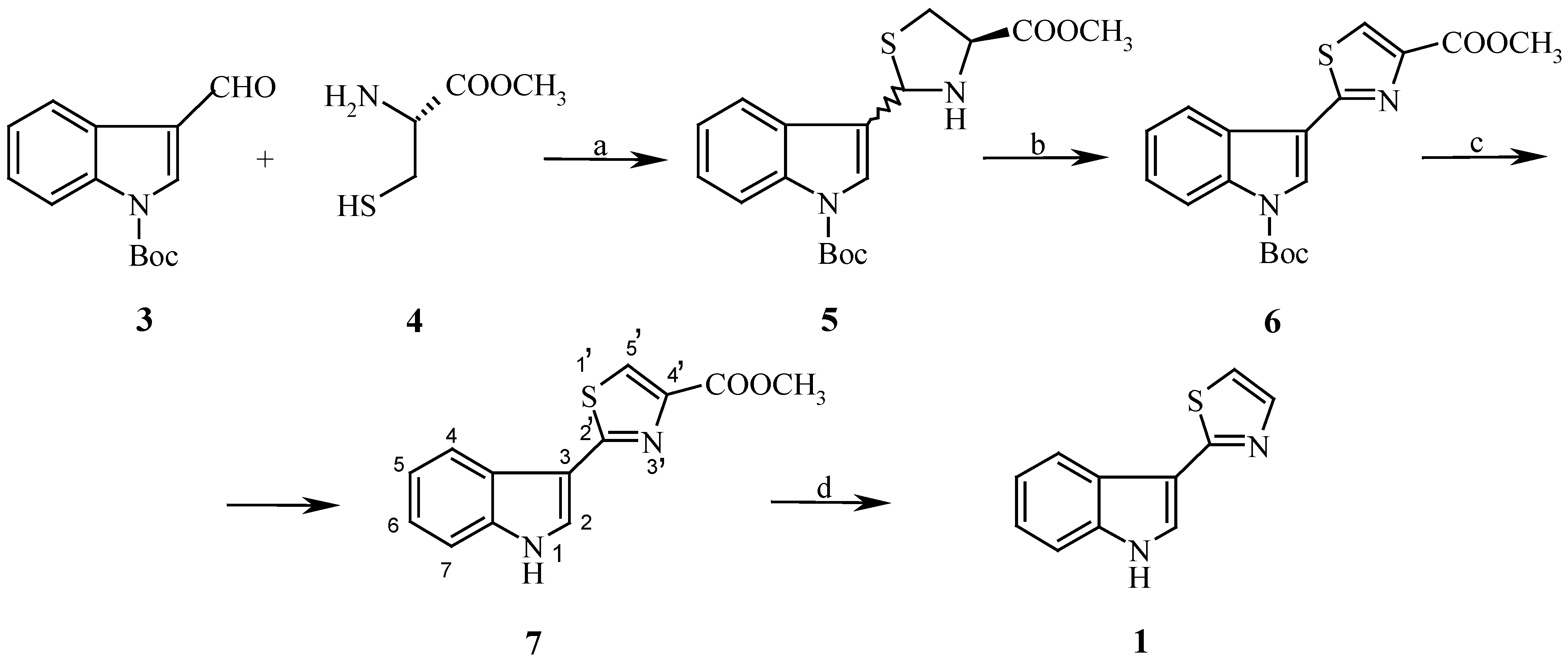
3) was prepared according to the described procedure [
11].
1-(tert-Butoxycarbonyl)-4´-methoxycarbonyl-3-(thiazolidin-2´-yl)indole (5).
To a suspension of methyl L-cysteinate hydrochloride (595 mg, 3.47 mmol) in 1:2 methanol/benzene (6mL) was added 1-(tert-butoxycarbonyl)indole-3-carboxaldehyde (490 mg, 2 mmol) and triethylamine (672 mg, 6.6 mmol). The reaction mixture was stirred for 3 hours at room temperature, the solvent was evaporated and the oily residue purified by column chromatography, using a mixture of cyclohexane/acetone (2:1) as eluent. Yield 615 mg (85%), yellow oil; For C18H22N2O4S (362.50) calculated: 59.65% C, 6.12% H, 7.73% N; found: 59.60% C, 6.07% H, 7.65% N; IR: 3323 (NH), 1720 a 1730 (C=O); 1H-NMR (ppm): 1,66 s, 9H [(CH3)3], 2,96 s 1H (NH), 3,09-4,33 m, 3H (SCH2CH), 3,79 s, 3H (OCH3), 5,78 s a 6,01 s 57:43, 1H (CH) , 7,13-8,22 m, 5H (H-arom.).
1-(tert-Butoxycarbonyl)-4´-methoxycarbonyl-3-(thiazol-2´-yl) indole (6).
To a stirred suspension of activated MnO
2 [
10] (1000 mg, 11.5 mmol) in a mixture of dry benzene (10mL) and pyridine (0.050 mL) was added a solution of thiazolidine
5 ( 400 mg, 1.10 mmol) in dry benzene (2 mL). The reaction mixture was stirred for 1.5 hour at 55
o C. After cooling the insoluble material was removed by filtration, washed with benzene, the solvent was evaporated and the solid residue crystallized from a mixture of diethylether/hexane. Yield 175 mg (44%), M.p. 128-130
o C; For C
18H
18N
2O
4S (359.48) calculated: 60.14% C, 5.05% H, 7.79% N; found: 60.23% C, 5.21% H, 7.29% N; IR: 3323 (NH), 1720 a 1730 (C=O);
1H-NMR (ppm): 1,66 s, 9H [(CH
3)
3], 3.79 s, 3H (OCH
3), 8,10 s, 1H (H-2), 8,25 s, 1H (H-5´), 7.13-8,22 m, 4H (H-arom.).
4´-Methoxycarbonyl-3-( thiazol-2´-yl) indole (7).
To a suspension of thiazole 6 (150 mg, 0.42 mmol) in dry methanol (12mL) was added sodium methoxide (330 mg, 6.11 mmol) during 5 min. The reaction mixture was poured into cold water (60 mL), extracted with chloroform (3x10 mL), dried over Na2SO4, the solvent evaporated and the product crystallized from a mixture of diethylether/hexane. Yield 64 mg (59%), M.p. 166-168o C; For C13H10N2O2S (259.50) calculated: 60.17% C, 3.88% H, 10.80% N; found: 60.28% C, 3.99% H, 10.95% N; IR: 3200 (NH), 1720 a 1730 (C=O); 1H-NMR (ppm): 3,79 s, 3H (OCH3), 8.10 d, 1H (H-2), J=2,55 Hz, 8,25 s, 1H (H-5´), 7.13-8.22 m, 4H (H-arom.), 10,85 s, 1H (NH). 13C NMR (ppm): 52,32 (CH3), 113.05, 121.66, 122.04, 123.83, 125.50, 125.72, 127.41, 127.58, 137.99, 147.78 (C arom.), 162.61 (C=N), 164.75 (C=O). MS, m/z (%), : 258 (M+, 100), 200 (24), 160 (24), 142 (24), 115 (12), 57 (12).
Camalexin (1).
To a solution of NaOH (22.0 mg, 5.6 mmol) in water (2 mL) was added a solution of thiazole
7 (120 mg, 0.46 mmol) in methanol (2 mL) and the reaction mixture was refluxed for 1 hour. After cooling and evaporation of the methanol, NaHCO
3 (660 mg, 7.86 mmol) was added and the reaction mixture was refluxed for 1 hour. The product separated after cooling and was collected on filter paper and dried. Crystallization from a mixture of diethylether/hexane yielded 10 mg (12%); M.p. 140-141
o C; For C
11H
8N
2S (200.10) calculated: 66.00% C, 4.00% H, 14.00% N; found: 65.80% C, 4.00% H, 13.50% N; IR,
1H-,
13C-NMR and mass spectra were identical with previously described data for camalexin [
1,
3,
6].