Introduction
Synthetic podands are a family of linear multidentate ligands, which includes acyclic polyethers. They generally form complexes with smaller stability constants than those of corresponding macrocyclic complexes and thus are usually regarded as poor ligands [
2]. In contrast, many excellent podands are found in nature. For example, naturally occurring polyether antibiotics, such as monsin and lasalocid, selectively bind several metal cations and effectively transport them across a biomembrane [
3]. Synthetic podands have advantages over these biological podands, in terms of facile synthesis and versatility of molecular structure [
2].
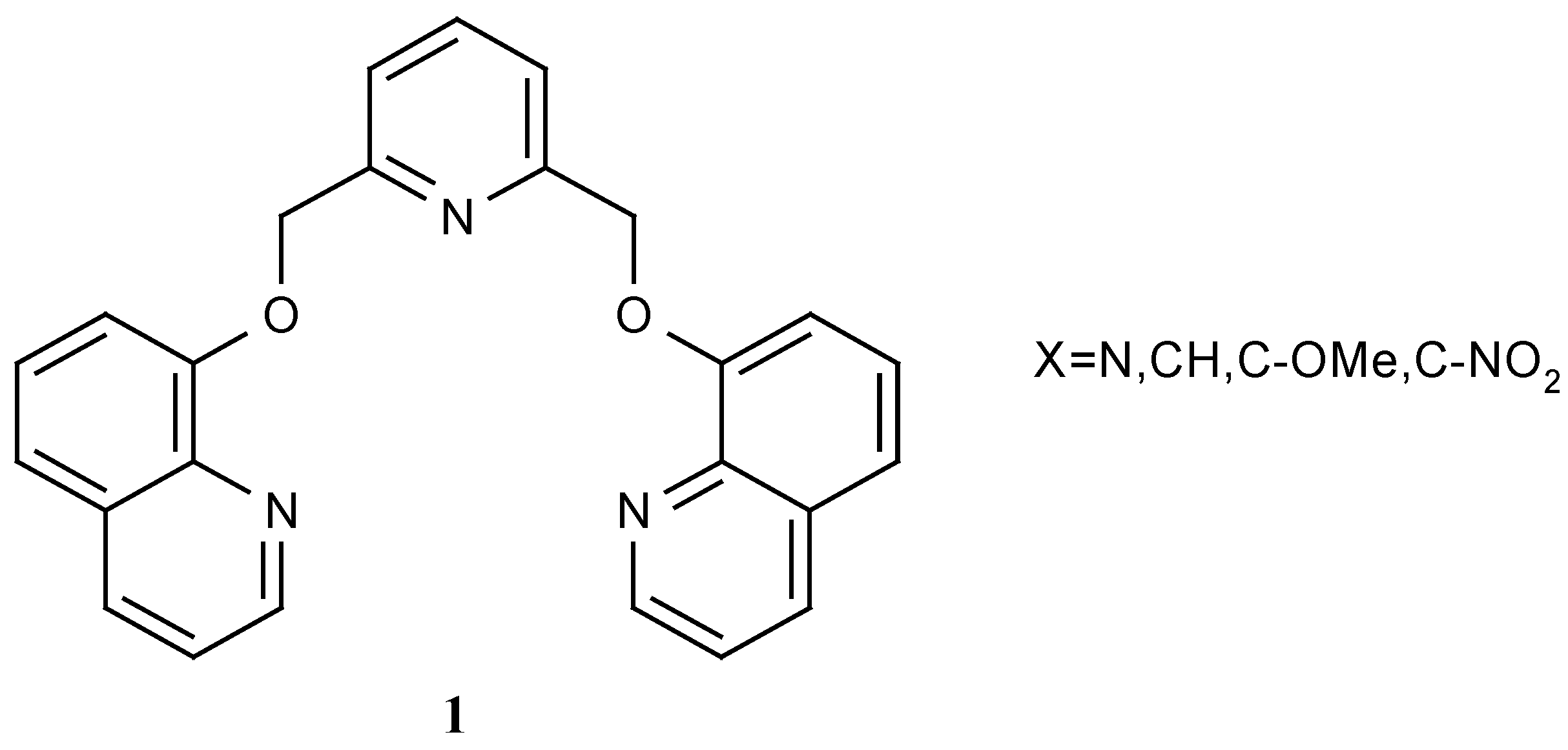
Vogtel and Weber, have reported a variety of podands having quinoline “end groups” (
Figure 1) [
4,
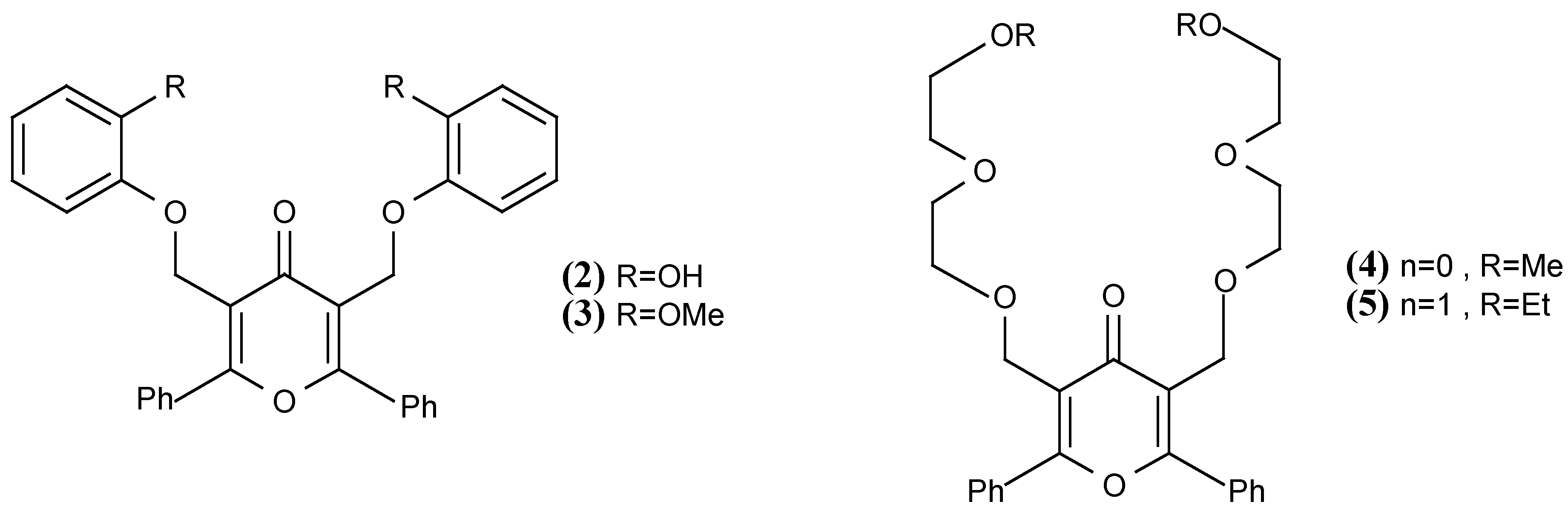
5]. Recently we have reported the synthesis of some pyrone podands such as 2 - 5 (
Figure2)[
6].
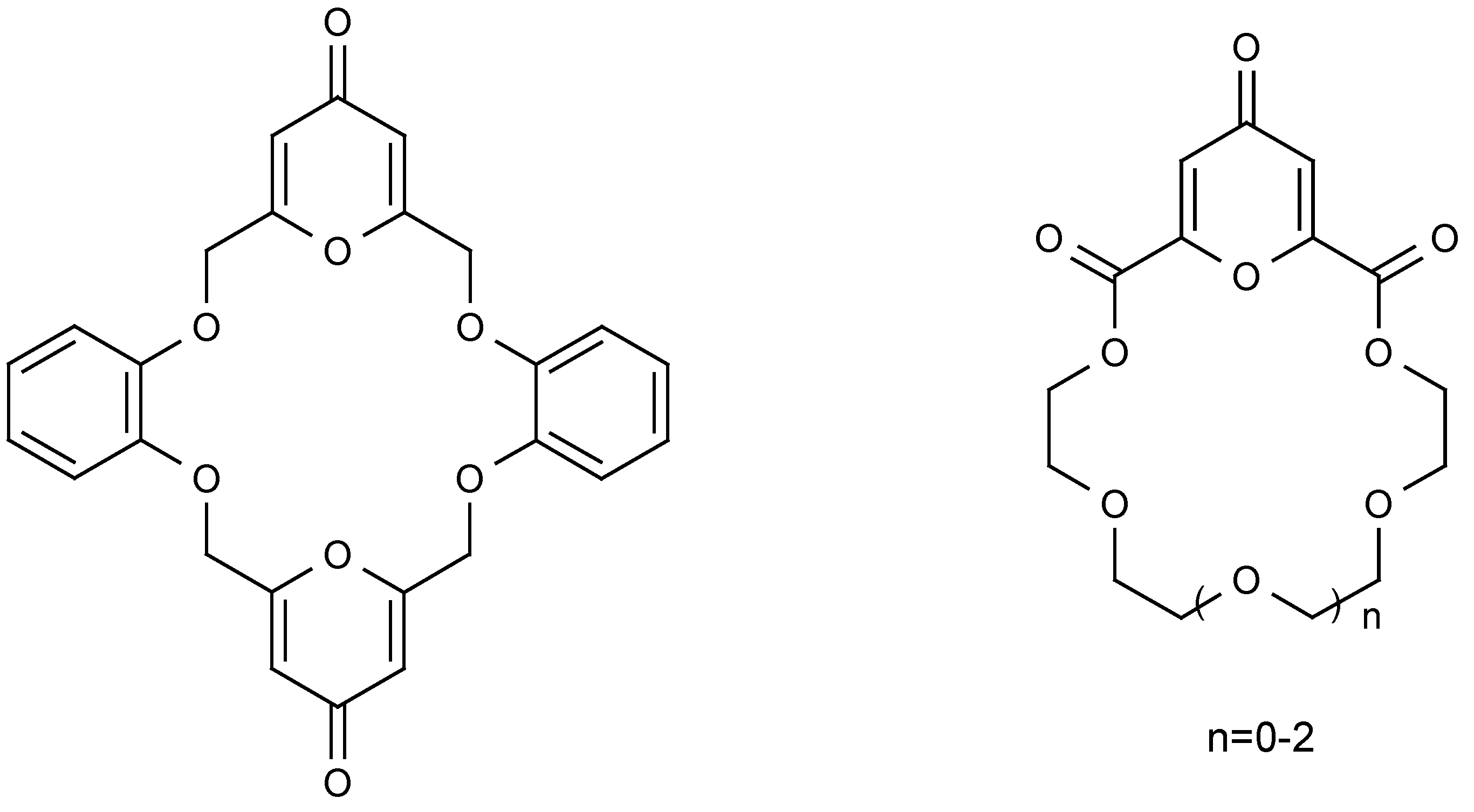
Furano-, pyrano-, and thiopheno- derivatives are well known crown ether type compounds [
7,
8]. To our knowledge, two papers have been published concerned with crown ethers and crown ether diesters possessing 4
H-pyran-4-one subcyclic unit(s) (
Figure 3) [
9,
10].
However, synthesis of 4H-pyran-4-one podands possessing nitrogen atoms, has not been reported as yet. As a continuation of our investigations on the chemistry of pyrones, we now report the synthesis of some new podands and crown ethers derived from 3,5-disubstituted 4H-pyran-4-one.
Experimental
General
Melting points were determined with an Electrothermal Instrument model 9100 and are uncorrected. Infrared (IR) spectra were run on a Shimadzu IR 435 Spectrophotometer as KBr disks or as smears between salt plates. The 1H-NMR spectra were recorded on a Varian-EM 390 spectrometer. The 13C-NMR spectra were recorded on a FT-NMR Brucker 80 MHz spectrometer. Chemical shifts are reported in ppm with TMS as internal standard. Mass spectra were taken with a Varian Mat 711 double focusing mass spectrometer. Elemental analyses were performed on a Heareus, CHN-O-RAPID analyzer.
General Procedure for the Reactions of Pyrone 6 with Salicylaldehyde, 2-Nitrophenol and 8-Hydroxyquinoline: Preparation of Compounds 7-9.
To a solution of salicylaldehyde (or 2-nitrophenol or 8-hydroxyquinoline, 0.335g , 2.76 mmol) in alcohol (3 mL) was added sodium hydroxide (0.1g, 2.79 mmol) in water (40mL). The mixture was warmed and pyrone 6 (0.6 g, 6.9 mmol) was added. Sufficient ethyl alcohol to produce a homogeneous solution was then added. The solution was refluxed under nitrogen for 12 hr and cooled to 0°C and then filtered. The crystals were washed with water and dried in a desiccator.
3,5-Bis (2-nitrophenoxymethyl)-2,6-diphenyl-4H-pyran-4-one (8).
The crude product was recrystallized from CHCl3/EtOH to give compoud 8 in 47% yield, mp: 235-236oC; 1H-NMR (CDCl3) δ: 5.1 (4H, s), 6.9-8 (18H, m); 13C-NMR (CDCl3) δ: 63.93, 116.62, 118.98, 121.06, 121.10, 125.46, 128.95, 129.00, 131.24, 131.30, 134.25, 152.17, 166.79, 178.03; IR (KBr) cm-1: 1645, 1600, 1515, 135, 1240; Analysis: Calc. for C31H22N2O8: C 67.63, H 4.03, N 5.09; Found: C 67.26, H 4.31, N 4.67.
3,5-Bis (8-qinolinoxymethyl)-2,6-diphenyl-4H-pyran-4-one (9).
The crude product was purified by dry column chromatography on silicagel using ethyl acetate-petroleum ether (1:1) as eluent to give compound 9 in 54% yield, mp: 249-2500C; 1H-NMR (CDCl3) δ: 5.18 (4H, s), 7.25-7.95 (18H, m), 8.15 (2H, dd), 9.01 (2H, dd); 13C-NMR (CDCl3) δ: 63.06, 110.64, 120.28, 120.49, 121.85, 127.11, 128.96, 129.42, 129.90, 131.24, 132.28, 136.17, 141.08, 149.73, 155.06, 166.38, 178.74; IR (KBr) cm-1: 3050, 2918, 1645, 1615, 1560, 1490, 1445, 1410, 1310, 1250, 1160, 1090, 895, 700; MS (m/z): 562, 418, 273, 159, 105, 77; Analysis: Calc. for C37H26N2O4: C 78.99, H 4.66, N 4.98; Found: C 78.66, H 4.42, N 4.57.
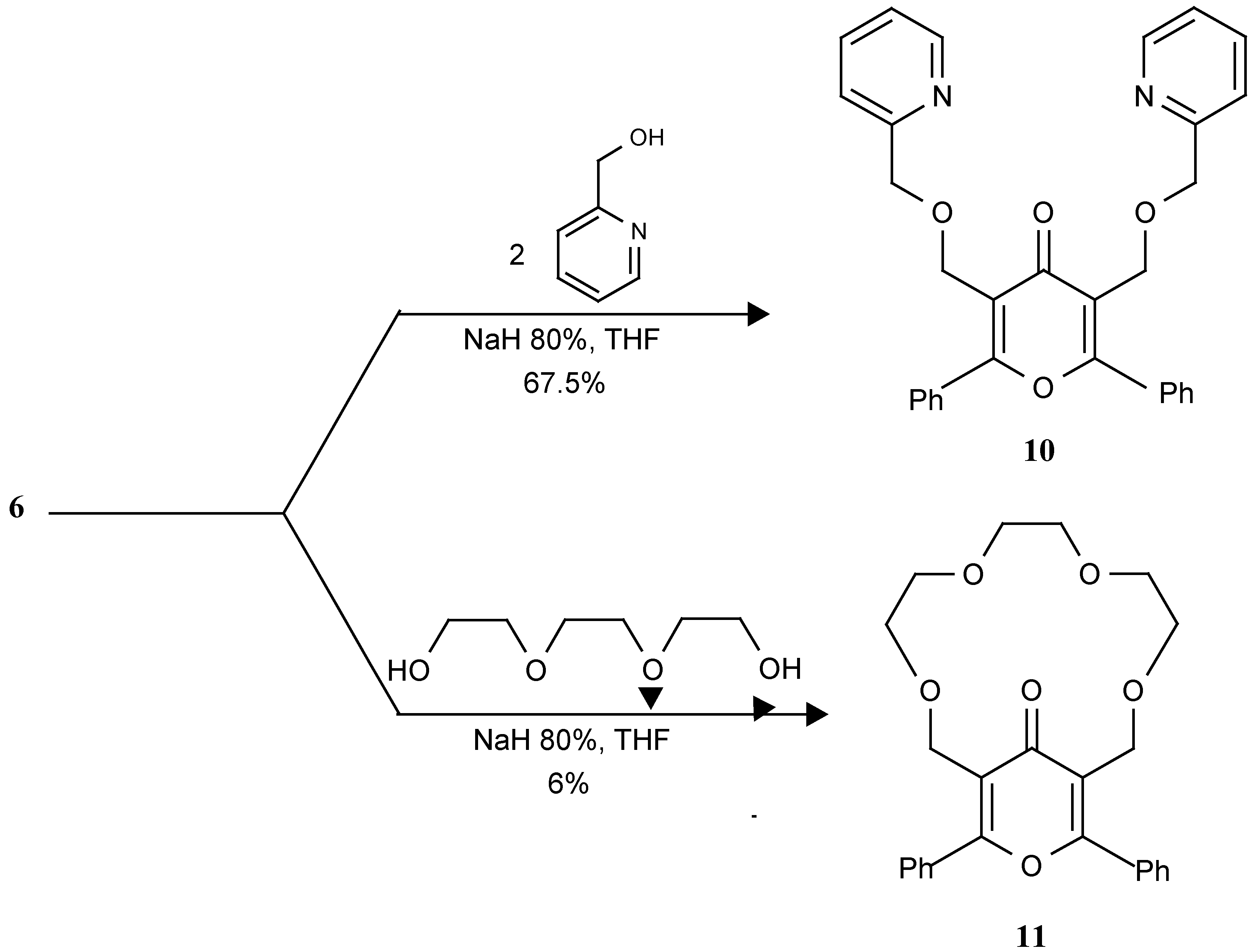
3,5-Bis-(2-pyridylmethoxymethyl)-2, 6-diphenyl-4H-pyron-4-one (10).
A mixture of 2-hydroxymethyl pyridine (0.22 ml, 2.3 mmol), sodium hydride (80% in mineral oil; 0.1g, 3.45 mmol) and anhydrous THF (70 mL) was stirred at room temperature (rt) under nitrogen for 10 min and then refluxed for 60 min. After cooling to rt, pyrone 6 (0.5g, 1.15 mmol) in anhydrous THF (30 mL) was added over 15 min. The resulting mixture was stirred at rt. for 18 hr and then water was added (15mL) and the pH adjusted to 7 with dilute HCl. Then 70mL of solvent were removed under reduced pressure and the residue was extracted with CH2Cl2. (3×50 mL). The combined CH2Cl2 extracts were dried over MgSO4 and solvent was removed under vacuum. The crude product was purified by column chromatography on silicagel using 9:1 ethyl acetate-methanol as eluent to give compound 10 in 67.5% yield, mp:149-150 oC; 1H-NMR (CDCl3) δ: 4.62 (4H, s), 4.85 (4H, s), 7.1-7.85 (16H, m), 8.58-8.9 (2H, m); 13C-NMR (CDCl3) δ: 64.36, 74.28, 121.35, 122.32, 122.76, 128.97, 129.24, 131.28, 132.39, 137.13, 149.29, 158.84, 165.54, 179.36; IR (KBr) cm-1:3050, 2850, 2750, 1640, 1615, 1580, 1440, 1410, 1330, 1250, 1095, 1080, 750, 690; MS (m/z): 490, 274, 273, 115, 105, 77. Analysis: Calc. for C31H26N2O4: C 75.90, H 5.34, N 5.71; Found: C 75.52, H 5.16, N 5.53.
15,17-Diphenyl-3,6,9,12,16-pentaoxabicyclo[12.3.1]octadeca-1 (17),14-dien-18-one (11).
To refluxing NaH (80%) (0.1 gr., 3.45 mmol), in THF (70 mL) was added triethylenglycol (0.168g, 1.15 mmol ) in THF (50 mL) and 3,5-bis (bromomethyl) 2,6-diphenyl-4H-pyran-4-one (6) (0.5 gr, 1.15 mmol) in THF (50 mL) using an Aldrich high dilution adapter (reactants added dropwise from an addition funnel are prediluted with approximately 20 mL of refluxing solvent held in a reservoir by an internal collar) over 5 hr. This mixture was stirred at rt under nitrogen for 14 hr and water (10 mL) was added. The pH was adjusted to 7 with dilute HCl, then 150mL of solvent were removed under reduced pressure and the residue extracted with CH2Cl2 (3x50 mL). The combined CH2Cl2 extracts were dried over MgCl2 and solvent was removed under vacuum. The crude product was purified by column chromatography on silicagel using 2:9 ethyl acetate-CHCl3 and then ethyl acetate as eluents to give compound 11 in 6% yield, mp: 116 - 117˚C; 1H-NMR (CDCl3) δ: 3.6-3.8 (12H, m), 4.5 (4H, s), 7.35-7.75 (10H, m); IR cm-1: 2992, 2883, 1657, 1418, 1094, 1024, 708; MS (m/z): 422, 378, 289, 273, 159, 105, 77.