Unambiguous Assignment of the 1H- and 13C-NMR Spectra of Propafenone and a Thiophene Analogue
Abstract
:Introduction
Results and Discussion

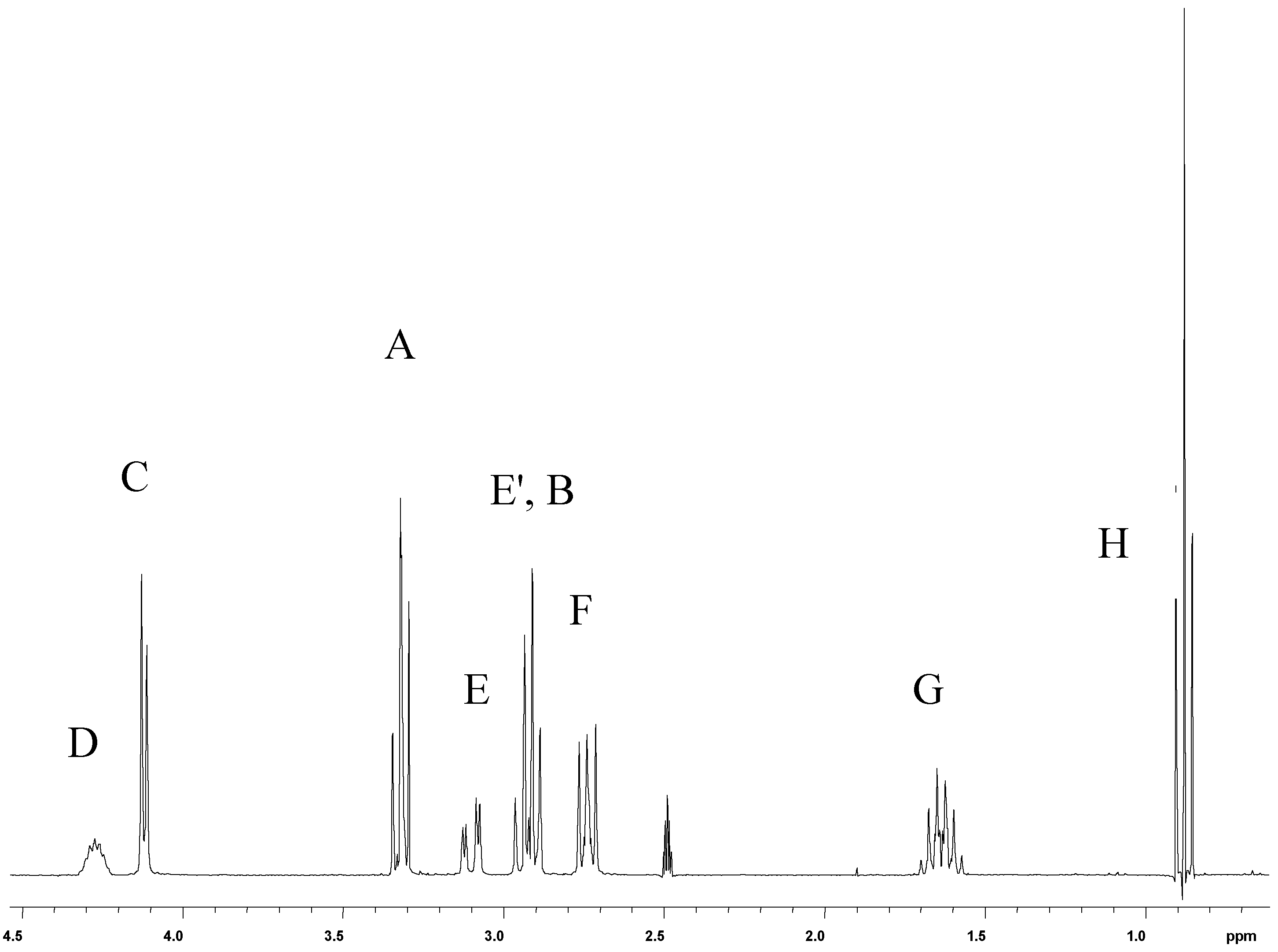
1H-NMR Spectra
13C-NMR Spectra
Conclusions
Experimental
| Compound | 1 | 1•HCl | 2 | 2•HCl | ||||
|---|---|---|---|---|---|---|---|---|
| Solvent | DMSO-d6 | CDCl3 | DMSO-d6 | CDCl3 | DMSO-d6 | CDCl3 | DMSO-d6 | CDCl3 |
| 'aromatic' H-atoms' | ||||||||
| 3 | 7.14 | 6.96 | 7.16 | 6.99 | 7.12 | 6.85 | 7.15 | 6.87 |
| 4 | 7.49 | 7.42 | 7.50 | 7.50 | 7.86 | 7.48 | 7.86 | 7.44 |
| 5 | 6.99 | 7.00 | 7.01 | 7.05 | --- | --- | --- | --- |
| 6 | 7.52 | 7.65 | 7.53 | 7.74 | --- | --- | --- | --- |
| 2',6' | 7.22 | 7.24 | 7.21-7.28 | 7.21 | 7.24 | 7.25 | 7.23-7.30 | 7.24 |
| 3',5' | 7.23 | 7.27 | 7.21-7.28 | 7.30 | 7.25 | 7.28 | 7.23-7.30 | 7.28 |
| 4' | 7.15 | 7.17 | 7.15 | 7.20 | 7.16 | 7.18 | 7.15 | 7.15 |
| ‘aliphatic' H-atoms | ||||||||
| A | 3.33 | 3.33 | 3.33 | 3.32 | 3.21 | 3.23 | 3.22 | 3.13 |
| B | 2.90 | 3.03 | 2.91 | 3.01 | 2.90 | 3.02 | 2.92 | 2.99 |
| C, C' | 4.08, 4.01 | 4.06 | 4.14 | 4.20 | 4.19, 4.13 | 4.14, 4.13 | 4.26 | 4.27, 4.22 |
| D | 3.90 | 4.00 | 4.32 | 4.54 | 3.86 | 3.96 | 4.26 | 4.60 |
| E, E' | 2.64, 2.58 | 2.79, 2.69 | 3.11, 2.94 | 3.43, 3.14 | 2.61, 2.56 | 2.78, 2.69 | 3.11, 2.93 | 3.27, 3.13 |
| F | 2.41 | 2.53 | 2.74 | 2.94 | 2.39 | 2.51 | 2.74 | 2.88 |
| G | 1.37 | 1.49 | 1.66 | 1.97 | 1.36 | 1.46 | 1.65 | 1.88 |
| H | 0.83 | 0.91 | 0.87 | 1.01 | 0.83 | 0.91 | 0.88 | 0.96 |
| NH and OH | * | 2.70 (2H) | 9.15 (2H), 5.96 | 9.40, 8.84, 1.65 | 4.99 (1 H) | 2.15 (2H) | 9.13 (2H), 5.90 | 9.28, 8.70 |

| Compound | 1 | 1•HCl | 2 | 2•HCl | |||||
|---|---|---|---|---|---|---|---|---|---|
| Solvent | DMSO-d6 | CDCl3 | * | DMSO-d6 | CDCl3 | DMSO-d6 | CDCl3 | DMSO-d6 | CDCl3 |
| sp2-hybridized C-atoms | |||||||||
| 1 | 128.0 | 128.4 | 125.5 | 128.0 | 128.0 | 121.5 | 122.9 | 121.7 | 121.4 |
| 2 | 157.5 | 157.6 | 159.1 | 157.1 | 157.1 | 159.8 | 159.2 | 159.0 | 159.3 |
| 3 | 113.1 | 113.0 | 115.9 | 113.2 | 113.2 | 118.1 | 116.9 | 118.0 | 117.1 |
| 4 | 133.4 | 133.3 | 132.5 | 133.5 | 134.5 | 133.7 | 132.5 | 133.4 | 132.2 |
| 5 | 120.4 | 121.0 | 121.4 | 120.7 | 121.3 | --- | --- | --- | --- |
| 6 | 129.5 | 130.2 | 130.7 | 129.5 | 130.9 | --- | --- | --- | --- |
| 1' | 141.3 | 141.5 | 140.7 | 141.3 | 141.0 | 141.3 | 141.6 | 141.2 | 141.2 |
| 2',6' | 128.2 | 128.3 | 128.7 | 128.3 | 128.4 | 128.2 | 128.3 | 128.1 | 128.4 |
| 3',5' | 128.1 | 128.3 | 128.4 | 128.2 | 128.6 | 128.1 | 128.3 | 128.0 | 128.5 |
| 4' | 125.6 | 125.9 | 126.3 | 125.7 | 126.2 | 125.7 | 125.9 | 125.5 | 126.1 |
| C=O | 201.1 | 201.5 | 201.8 | 201.0 | 201.7 | 191.3 | 192.1 | 191.1 | 192.0 |
| sp3-hybridized C-atoms | |||||||||
| A | 44.5 | 45.1 | 41.3 | 44.4 | 43.4 | 42.1 | 43.1 | 41.9 | 43.0 |
| B | 29.7 | 30.2 | 30.6 | 29.7 | 30.2 | 29.5 | 30.1 | 29.3 | 30.1 |
| C | 71.2 | 71.4 | 72.0 | 70.4 | 71.5 | 74.4 | 74.0 | 73.4 | 74.2 |
| D | 67.9 | 67.8 | 69.3 | 64.8 | 64.8 | 68.1 | 67.7 | 64.8 | 65.0 |
| E | 52.3 | 51.8 | 52.4 | 49.8 | 51.6 | 52.1 | 51.5 | 48.7 | 51.5 |
| F | 51.2 | 51.6 | 52.3 | 48.8 | 50.7 | 51. 3 | 51.5 | 48.7 | 50.6 |
| G | 22.5 | 23.1 | 23.1 | 18.7 | 19.4 | 22.7 | 23.2 | 18.5 | 19.3 |
| H | 11.6 | 11.6 | 11.9 | 10.9 | 11.2 | 11.7 | 11.6 | 10.7 | 11.1 |

References and Notes
- Petrik, W.; Sachse, R. (Helopharm) Verfahren zur Herstellung neuer, therapeutisch wertvoller Derivate des 2'-Hydroxy-3-phenyl-propiophenons und deren Salze. DE 2001431, Jan. 6, 1970. Chem. Abstr. 1971, 75, 151538f. [Google Scholar]
- Bryson, H. M.; Palmer, K. J.; Langtry, H. D.; Fitton, A. Propafenone. A Reappraisal of its Pharmacology, Pharmacokinetics and Therapeutic Use in Cardiac Arrhythmias. Drugs 1993, 45, 85–130. [Google Scholar]
- Chiba, P.; Ecker, G.; Tell, B.; Moser, A.; Schmid, D.; Drach, J. Modulation of PGP-Mediated Multidrug-Resistance by Propafenone Analogs. Proc. Am. Assoc. Cancer Res. 1994, 35, 357. [Google Scholar]
- Ecker, G.; Fleischhacker, W.; Chiba, P. Pharmakologisch wirksame o-Acylaryloxypropanolamine mit tertiärem und quartärem Stickstoff. Austrian Patent Appl. 15A 963/93-1, 17 May 1993. [Google Scholar]
- Chiba, P.; Burghofer, S.; Richter, E.; Tell, B.; Moser, A.; Ecker, G. Synthesis, Pharmacologic Activity, and Structure-Activity Relationships of a Series of Propafenone-Related Modulators of Multidrug Resistance. J. Med. Chem. 1995, 38, 2789–2793. [Google Scholar] [CrossRef] [PubMed]
- Ecker, G.; Chiba, P.; Hitzler, M.; Schmid, D.; Visser, K.; Cordes, H. P.; Csöllei, J.; Seydel, J. K.; Schaper, K.-J. Structure-Activity Relationship Studies on Benzofuran Analogs of Propafenone-Type Modulators of Tumor Cell Multidrug Resistance. J. Med. Chem. 1996, 39, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Studenik, C.; Lemmens-Gruber, R.; Heistracher, P.; Ecker, G.; Maxl, A.; Fleischhacker, W. Electromechanical Effects of Newly Synthetized Propafenone Derivatives on Isolated Guinea-Pig Heart Muscle Preparations. Arzneim.-Forsch./Drug Res. 1996, 46(I), 134–138. [Google Scholar]
- Sánz-García, T.; González-Gaitano, G.; Iza, N.; Gálvez-García, A.; Tardajos, G. NMR Study of the Inclusion Complex between β-Cyclodextrin and Propafenone. In Spectroscopy of Biological Molecules: New Directions, 8th European Conference of Biological Molecules September 1999; Kluwer Academic Publishers: Dodrecht, 1999; pp. 333–334. [Google Scholar]
- Binder, D.; Noe, C. R.; Holzer, W. Ein Thiophenanalogon des Propafenons. Arch. Pharm. (Weinheim) 1990, 323, 919–921. [Google Scholar] [CrossRef]
- Patt, S. L.; Shoolery, J. N. Attached Proton Test for Carbon-13 NMR. J. Magn. Reson. 1982, 46, 535–539. [Google Scholar]
- Neuhaus, D.; Williamson, M. P. The Nuclear Overhauser Effect in Structural and Conformational Analysis; VCH Publishers: New York - Weinheim - Cambridge, 1989; pp. 211–252. [Google Scholar]
- Bax, A.; Freeman, R. Investigation of Complex Networks of Spin-Spin Coupling by Two-Dimensional NMR. J. Magn. Reson. 1981, 44, 542–561. [Google Scholar]
- Bax, A.; Subramanian, S. Sensitivity-Enhanced Two-Dimensional Heteronuclear Shift Correlation NMR Spectroscopy. J. Magn. Reson. 1986, 67, 565–569. [Google Scholar]
- Davis, D. G.; Bax, A. Simplification of 1H NMR Spectra by Selective Excitation of Experimental Subspectra. J. Am. Chem. Soc. 1985, 107, 7197–7198. [Google Scholar] [CrossRef]
- Bax, A. Structure Determination and Spectral Assignment by Pulsed Polarization Transfer via Long-Range 1H-13C Couplings. J. Magn. Reson. 1984, 57, 314–318. [Google Scholar]
- Kalchhauser, H.; Robien, W. CSEARCH: A Computer Program for Identification of Organic Compounds and Fully Automated Assignment of Cabon-13 Nuclear Magnetic Resonance Spectra. J. Chem. Inform. Comput. Sci. 1985, 25, pp. 103–108, For more information and the performance of estimations see http://mailbox.univie.ac.at/~robienw8/csearch_server_info.html. [CrossRef]
- Sample Availability: Compound 2•HCl is available from MDPI.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Holzer, W. Unambiguous Assignment of the 1H- and 13C-NMR Spectra of Propafenone and a Thiophene Analogue. Molecules 2001, 6, 796-802. https://doi.org/10.3390/61000796
Holzer W. Unambiguous Assignment of the 1H- and 13C-NMR Spectra of Propafenone and a Thiophene Analogue. Molecules. 2001; 6(10):796-802. https://doi.org/10.3390/61000796
Chicago/Turabian StyleHolzer, Wolfgang. 2001. "Unambiguous Assignment of the 1H- and 13C-NMR Spectra of Propafenone and a Thiophene Analogue" Molecules 6, no. 10: 796-802. https://doi.org/10.3390/61000796




