New Conjugated Systems Derived from Piperazine-2,5-dione
Abstract
:Introduction

Results and Discussion
Symmetrical Bis-arylidene Derivatives

Mono Arylidene and Unsymmetrical Bis-arylidene Derivatives
Experimental
General
3,6-Di(3-thienylidene)piperazine-2,5-dione 5 and 3,6-Di(3-indolylidene)piperazine-2,5-dione 6
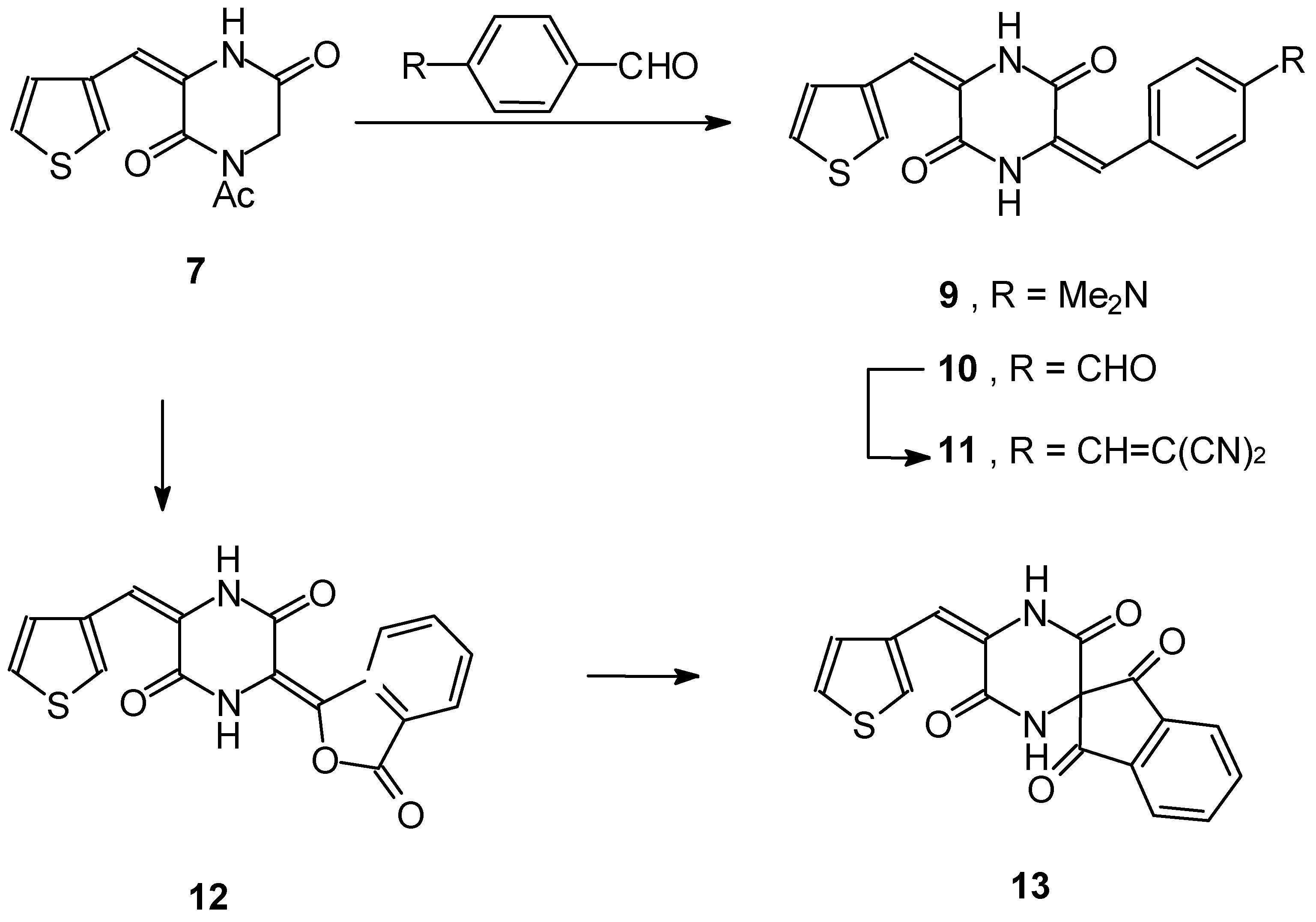
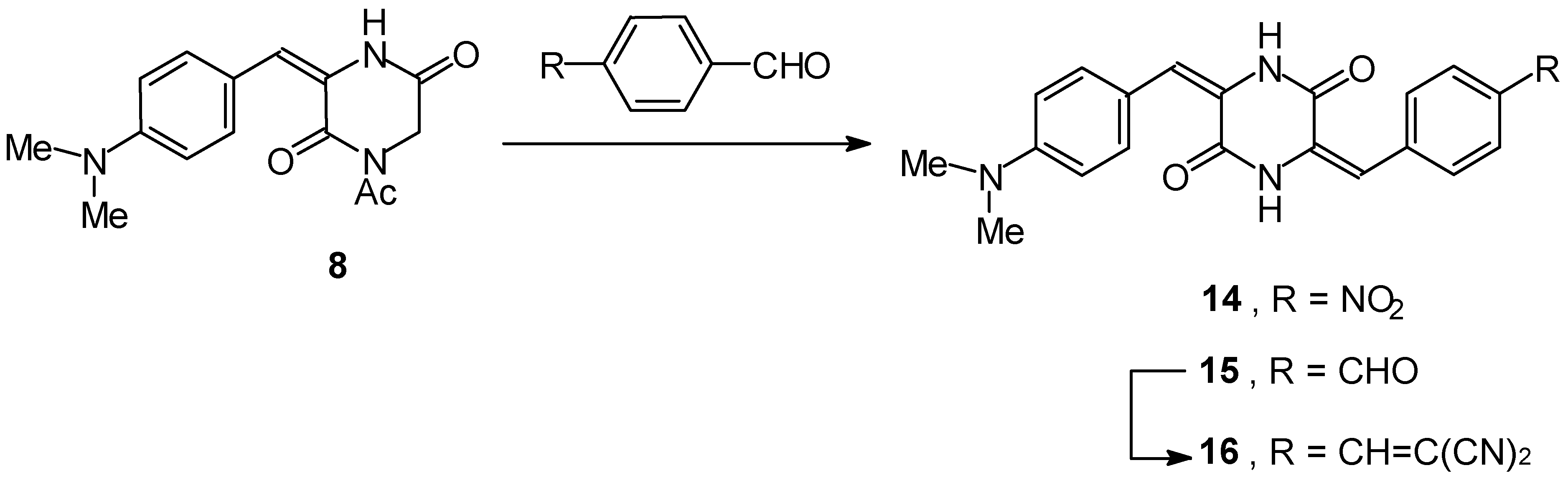
1-Acetyl-3-(3-thienylidene)piperazine-2,5-dione 7 and 1-Acetyl-3-(4-dimethylaminobenzylidene)pipe- razine-2,5-dione 8
General procedure for the preparation of unsymmetrical bisarylidenes:3-(3-Thienylmethylidene)-6-(4- dimethylaminobenzylidene)piperazine-2,5-dione 9, 3-(3-Thienylmethylidene)-6-(4-formylbenzylidene)- piperazine-2,5-dione 10, and 3-(3-Thienylmethylidene)-6-(1,3-dioxo-2-indanylidene)piperazine-2,5- dione 13
3-(4-Dimethylaminobenzylidene)-6-(4-nitrobenzylidene)piperazine-2,5-dione 14
3-(3-Thienylmethylidene)-6-[4-(1,1-dicyanovinylbenzylidene)]piperazine-2,5-dione 11
3-(4-Dimethylaminobenzylidene)-6-(4-formylbenzylidene)piperazine-2,5-dione 15
3-[4-(1,1-Dicyanocinyl)benzylidene]-6-(4-dimethylaminobenzylidene)piperazine-2,5-dione 16
References and Notes
- Sammes, P.G. Fortschr. Chem. Org. Naturst. 1975, 33, 51.
- Anteunis, M. J. O. Bull. Soc. Chim. Belg. 1978, 87, 625.
- Shin, C. Heterocycles 1983, 20, 1407.
- Schott, H. F.; Larkin, J. B; Rockland, L. B.; Dunn, M. S. J. Org. Chem. 1947, 12, 490. [PubMed]
- Blake, K.W.; Porter, A. E. A.; Sammes, P. G. J. Chem. Soc. Perkin Trans. 1 1972, 2494.
- Baxter, R. A.; Spring, F. S. J. Chem. Soc. 1947, 1179.
- Machin, P. J.; Porter, A. E.; Sammes, P. G. J. Chem. Soc. Perkin Trans. 1 1973, 404.
- Shin, C.; Nakajima, Y.; Sato, Y. Chem. Lett. 1984, 1301.
- Sakata, K.; Massgo, H.; Sakurai, A.; Takahashi, N. Tetrahedron Lett. 1982, 23, 2095.
- Shin, C.; Masaki, M.; Ohta, M. J. Org. Chem. 1967, 32, 1860.
- Marcuccio, S. M.; Elix, J. A. Aust. J. Chem. 1984, 37, 1791.
- Samples Availability: Available from the authors.

| Comp
No. | Yield
(%) | M.p.
(°C) | Colour of crystals | Molecular formula | Calculated (%) | Found (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | C | H | N | |||||
| 5 | 73 | > 340 | Yellow | C14H10N2O2S2 | 55.63 | 3.31 | 9.27 | 55.35 | 3.52 | 9.52 |
| 6 | 85 | > 340 | Yellow | C22H14N4O2 | 72.13 | 3.83 | 15.3 | 71.88 | 3.94 | 15.5 |
| 7 | 86 | > 320 | Yellow | C11H10N2O3S | 52.80 | 4.00 | 11.2 | 52.65 | 3.85 | 11.1 |
| 8 | 80 | > 320 | Yellow | C15H17N3O3 | 62.72 | 5.92 | 14.6 | 62.54 | 6.11 | 14.8 |
| 9 | 92 | > 320 | Yellow | C18H17N3O2S | 63.71 | 5.01 | 12.4 | 63.66 | 5.33 | 12.11 |
| 10 | 89 | > 300 | Yellow | C17H12N2O3S | 62.96 | 3.70 | 8.64 | 62.75 | 3.86 | 8.75 |
| 11 | 87 | > 340 | Dark Red | C20H12N4O2S | 64.52 | 3.22 | 15.05 | 64.32 | 3.42 | 15.38 |
| 13 | 92 | > 340 | Yellow | C17H10N2O4S | 60.36 | 2.96 | 8.28 | 60.22 | 3.12 | 8.42 |
| 14 | 94 | > 340 | Yellow | C20H18N4O4 | 63.49 | 4.76 | 14.82 | 63.22 | 4.85 | 14.95 |
| 15 | 75 | > 320 | Orange | C21H19N3O3 | 69.82 | 5.26 | 11.63 | 69.59 | 5.41 | 11.75 |
| 16 | 52 | >340 | Dark Red | C24H19N5O2 | 70.42 | 4.64 | 17.12 | 70.21 | 4.71 | 17.41 |
| Comp No. | δ | νmax/cm-1 | |||||
|---|---|---|---|---|---|---|---|
| NH | Ar-H +-CH=C- | Other | NH | C=O | C=C | Other | |
| 5 | 10.34 | 6.70-7.90 | 3266 | 1683 | 1625 | ||
| 6 | 10.84 | 7.10-8.40 | 3220 | 1702, 1665 | 1640 | ||
| 7 | 11.11 | 6.82-7.61 | 4.50 (s, 2H, CH2), 2.43 (s, 3H, CH3CO) | 3255 | 1693, 1661 | 1625 | |
| 8 | 10.34 | 7.00-7.55 | 4.42 (s, 2H, CH2), 3.01 (s, 6H, (CH3)2N), 2.49 (s, 3H, CH3CO) | 3320 | 1693, 1651 | 1609 | |
| 9 | 10.72 | 6.88-7.60 | 3.07 (s, 6H, (CH3)2N) | 3200 | 1683 | 1611 | 1705 (C=O) |
| 10 | 11.85 | 6.95-7.60 | 9.80 (s, 1H, CHO) | 3165 | 1694, 1682 | 1612 | 1705 (C=O) |
| 11 | 10.72 | 6.81-7.90 | 8.3 (s, 1H, CH=C(CN)2) | 3193 | 1688 | 1605 | 2197 (CN) |
| 13 | 10.85 | 6.66-8.20 | 3205 | 1670 | 1603 | 1695 (C=O) | |
| 14 | 10.89 | 6.82-8.60 | 3.1 (s, 6H, (CH3)2N) | 3225 | 1698, 1645 | ||
| 15 | 8.11 | 6.70-8.36 | 9.5 (s, 1H, CHO) | 3195 | 1687, 1652 | 1611 | 1698 (C=O) |
| 16 | 8.24 | 6.65-8.0 | 8.14 (s, 1H, CH=C(CN)2 | 3211 | 1682, 1650 | 1625 | 2197 (CN) |
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Asiri, A.M. New Conjugated Systems Derived from Piperazine-2,5-dione. Molecules 2000, 5, 629-636. https://doi.org/10.3390/50300629
Asiri AM. New Conjugated Systems Derived from Piperazine-2,5-dione. Molecules. 2000; 5(3):629-636. https://doi.org/10.3390/50300629
Chicago/Turabian StyleAsiri, Abdullah Mohamed. 2000. "New Conjugated Systems Derived from Piperazine-2,5-dione" Molecules 5, no. 3: 629-636. https://doi.org/10.3390/50300629




