Cycloaddition Reactions of C,N-Diphenylnitrone to Methylene-γ-butyrolactones
Abstract
:Introduction
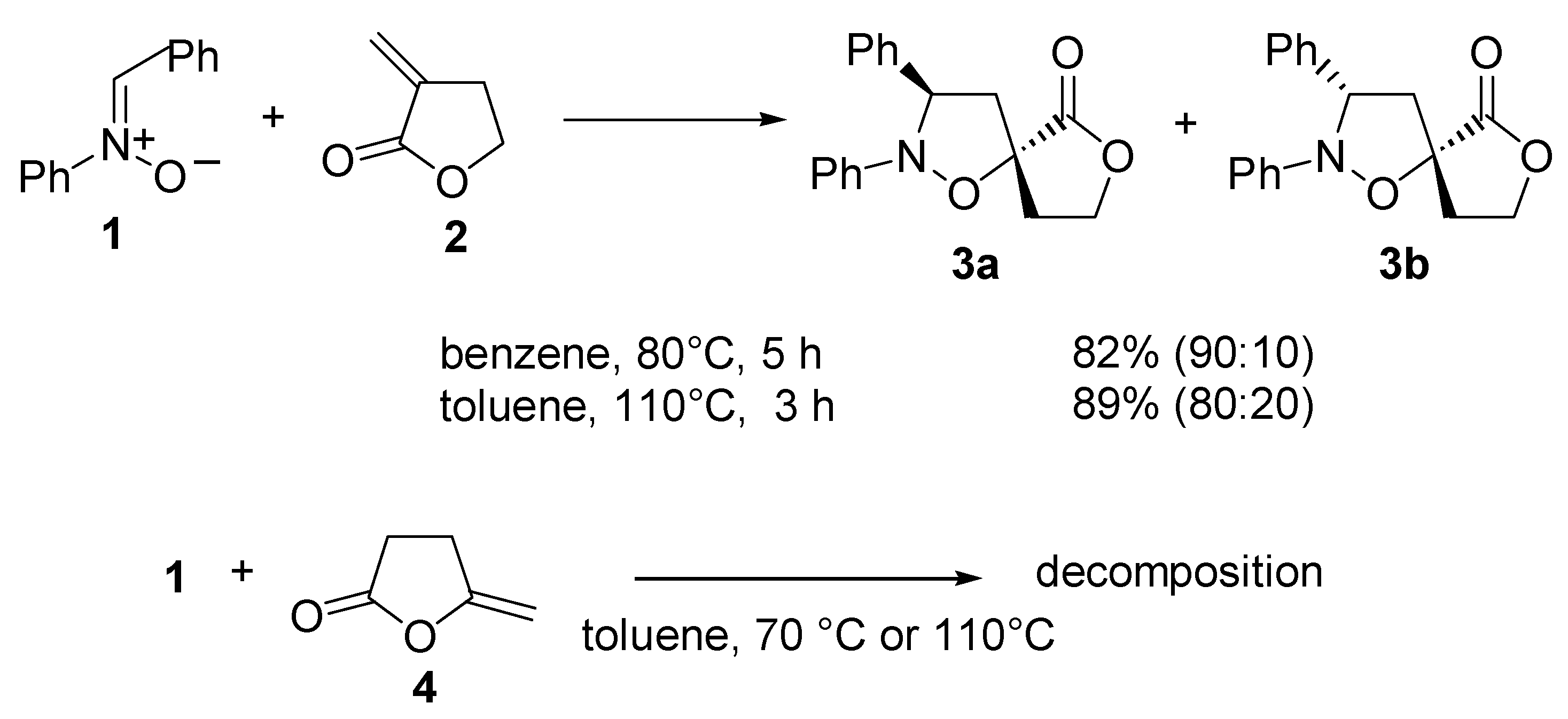
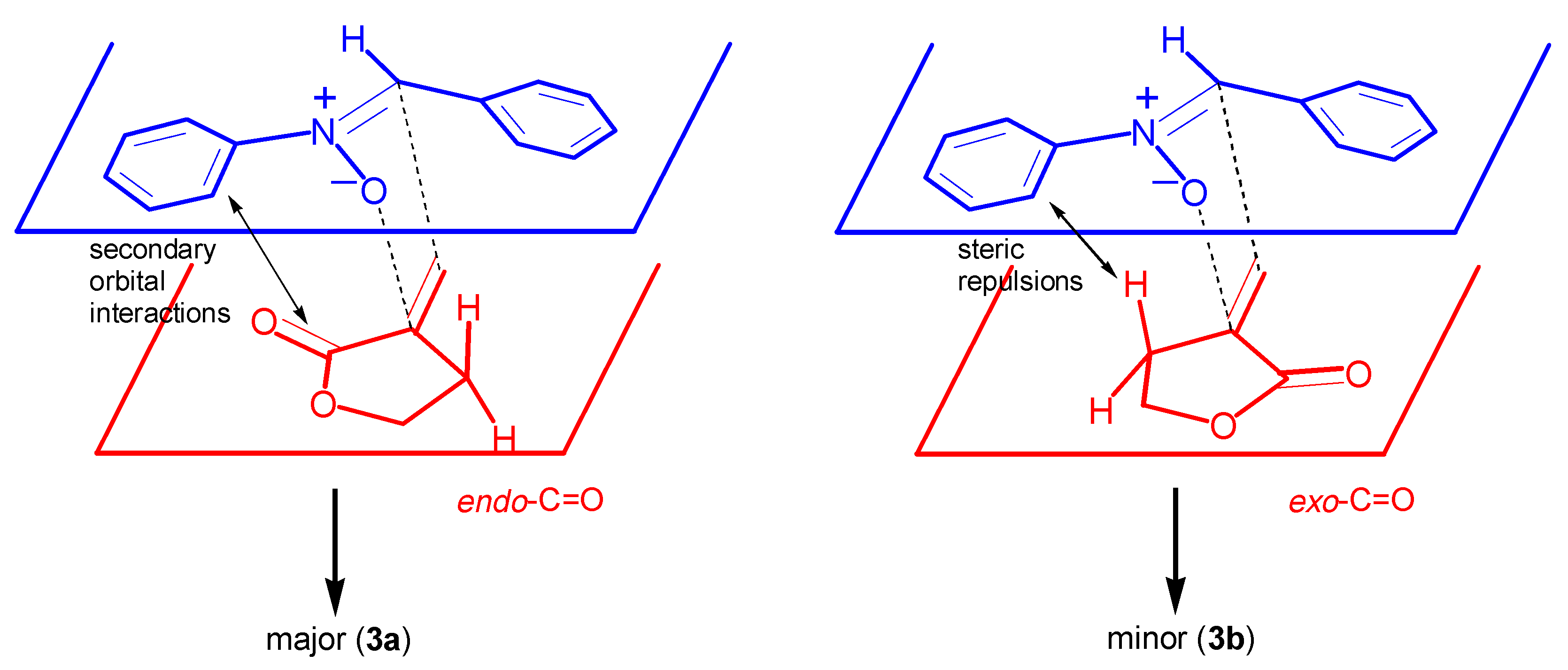
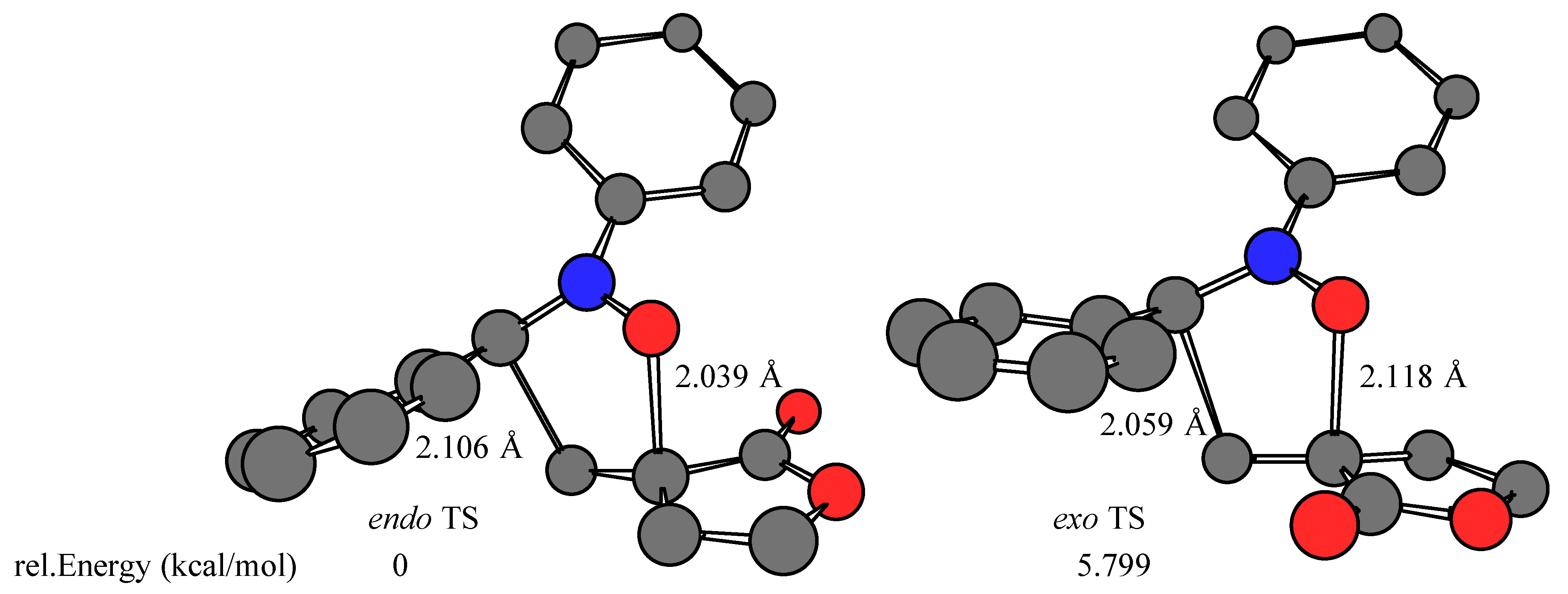
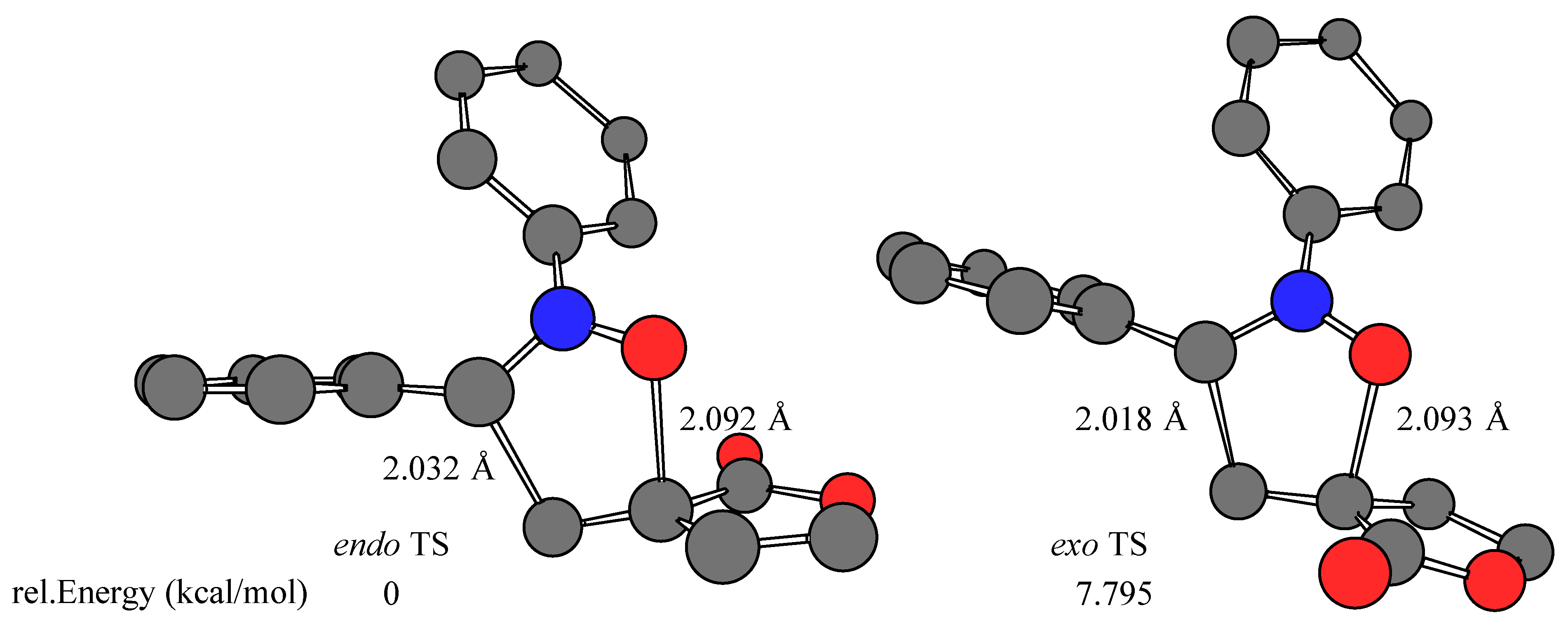
Results and Discussion
Conclusion
Experimental
General
Cycloaddition of C,N-Diphenylnitrone (1) to a-Methylene-y-butyrolactone (2) at 80°C
Cycloaddition of C,N-Diphenylnitrone (1) to a-Methylene-y-butyrolactone (2) at 110°C
Data of (3S*,3’R*)-Spiro[tetrahydrofuran-2-one-3,5’-(2’,3’-diphenyl)tetrahydroisoxazole] (3a)
Data of (3S*,3’S*)- Spiro[tetrahydrofuran-2-one-3,5’-(2’,3’-diphenyl)tetrahydroisoxazole] (3b)
X-Ray Structural Analysis of (3S*,3’R*)-Spiro[tetrahydrofuran-2-one-3,5’-(2’,3’-diphenyl)tetrahydro-isoxazole] (3a)
Acknowledgment
References and Notes
- Tufariello, J. J. 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; John Wiley & Sons: New York, 1984. [Google Scholar] Confalone, P. N.; Huie, E. M. Org. React. 1988, 36, 1–173. Torssell, K. B. G. Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis; Feuer, H., Ed.; VCH Publishers: New York, 1988. [Google Scholar] Döpp, D.; Döpp, H. Houben-Weyl - Methoden der organischen Chemie; Klamann, D., Hagemann, H., Eds.; Georg Thieme Verlag: Stuttgart, 1990; Vol. E14b. [Google Scholar] Breuer, E. The Chemistry of Amino, Nitroso and Nitro Compounds and Their Derivatives; Patai, S., Ed.; Wiley Interscience: New York, 1982. [Google Scholar] Frederickson, M. Tetrahedron 1997, 53, 403–425. Desimoni, G.; Tacconi, G.; Barco, A.; Pollini, G. P. Natural Product Synthesis Through Pericyclic Reactions; ACS Monograph n180; Caserio, M. C., Ed.; American Chemical Society: Washington, 1983. [Google Scholar]
- Brandi, A.; Cordero, F. M.; De Sarlo, F.; Goti, A.; Guarna, A. Synlett 1993, 1–8. Guarna, A.; Brandi, A.; De Sarlo, F.; Goti, A.; Pericciuoli, F. J. Org. Chem. 1988, 53, 2426–2429. Brandi, A.; Garro, S.; Guarna, A.; Goti, A.; Cordero, F.; De Sarlo, F. J. Org. Chem. 1988, 53, 2430–2434. Cordero, F. M.; Brandi, A.; Querci, C.; Goti, A.; De Sarlo, F.; Guarna, A. J. Org. Chem. 1990, 55, 1762–1767. Brandi, A.; Cordero, F. M.; Goti, A.; Guarna, A. Tetrahedron Lett. 1992, 33, 6697–6700. Cordero, F. M.; Anichini, B.; Goti, A.; Brandi, A. Tetrahedron 1993, 49, 9867–9876. Occhiato, E. G.; Guarna, A.; Brandi, A.; Goti, A.; De Sarlo, F. J. Org. Chem. 1992, 57, 4206–4211. Brandi, A.; Cicchi, S.; Cordero, F. M.; Frignoli, R.; Goti, A.; Picasso, S.; Vogel, P. J. Org. Chem. 1995, 60, 6806–6812. Zorn, C.; Goti, A.; Brandi, A.; Johnsen, K.; Noltemeyer, M.; Kozhushkov, S. I.; de Meijere, A. J. Org. Chem. 1999, 64, 755–763. Ferrara, M.; Cordero, F. M.; Goti, A.; Brandi, A.; Estieu, K.; Paugam, R.; Ollivier, J.; Salaün, J. Eur. J. Org. Chem. 1999, 2725–2739.
- Goti, A.; Cordero, F. M.; Brandi, A. Top. Curr. Chem. 1996, 178, 1–97. Brandi, A.; Goti, A. Chem. Rev. 1998, 98, 589–635.
- Goti, A.; Brandi, A.; De Sarlo, F.; Guarna, A. Tetrahedron 1992, 48, 5283–5300. Dapporto, P.; Paoli, P.; Brandi, A.; De Sarlo, F.; Goti, A.; Guarna, A. Acta Cryst. Sect. B 1992, 48, 234–238.
- Goti, A.; Anichini, B.; Brandi, A.; Kozhushkov, S.; Gratkowski, C.; de Meijere, A. J. Org. Chem. 1996, 61, 1665–1672. Zorn, C.; Anichini, B.; Goti, A.; Brandi, A.; Kozhushkov, S. I.; de Meijere, A.; Citti, L. J. Org. Chem. 1999, 64, 7846–7855.
- Goti, A. Tetrahedron 1996, 52, 9187–9192.
- de March, P.; Figueredo, M.; Font, J. Heterocycles 1999, 50, 1213–1226. Alonso-Perarnau, D.; de March, P; el Arrad, M.; Figueredo, M.; Font, J.; Parella, T. Tetrahedron 1997, 43, 14763–14772.
- For examples of cycloadditions of acyclic nitrones to: (a) an exo-methylene oxazolidinone, see: Pyne, S. G.; Safaei-G., J.; Skelton, B. W.; White, A. H. Aust. J. Chem. 1995, 48, 1511–1533. (b) an exo-methylene pyrrolidinone, see: Oravec, P.; Fišera, L.; Ertl Goljer, I. Monatsh. Chem. 1991, 122, 977–985. (c) an exo-methylene pyrrolidinthione, see: Fišera, L.; Jarošková, L.; Matejková, I; Heimgartner, H. Heterocycles 1995, 40, 271–278.
- Tufariello, J. J.; Tette, J. P. J. Org. Chem. 1975, 40, 3866–3869. Cid, P.; de March, P.; Figueredo, M.; Font, J.; Milán, S.; Soria, Á.; Virgili, A. Tetrahedron 1993, 49, 3857–3870. de Lange, B.; Feringa, B. L. Tetrahedron Lett. 1988, 29, 5317–5320. Banerji, A.; Basu, S. Tetrahedron 1992, 48, 3335–3344. Saito, S; Ishikawa, T; Kishimoto, N; Kohara, T; Moriwake, T. Synlett 1994, 282–284.
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; John Wiley: New York, 1976. [Google Scholar]
- DeShong, P.; Lander, S. W., Jr.; Leginus, J. M.; Dicken, C. M. Advances in Cycloaddition; Curran, D. P., Ed.; JAI Press: Greenwich, 1988; Vol. 1. [Google Scholar]
- Goti, A.; De Sarlo, F.; Romani, M. Tetrahedron Lett. 1994, 35, 6571–6574. [Google Scholar]
- The calculations were performed on a Silicon Graphics O2 R10000 using the Spartan software package (SPARTAN SGI Version 5.1.1; copyright® 1991-1998 by Wavefunction, Inc.). The transition structures were optimized at RHF/AM1 level in the gas phase (ΔHf = 39.643, 42.261, 40.033, 43.321 kcal/mol for Z-1 endo, Z-1 exo, E-1 endo, and E-1 exo TS, respectively). Single point energy RHF/3-21G(*) ab initio calculations (E = –605534.984, –605529.185, –605534.892, –605527.097 kcal/mol for Z-1 endo, Z-1 exo, E-1 endo, and E-1 exo TS, respectively) were carried out using the transition state geometries obtained at the RHF/AM1 level.
- Walker, N.; Stuart, D. Acta Cryst. Sect. A 1983, 39, 158–166.
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M. C.; Polidori, G.; Camalli, M. J. Appl. Cryst. 1994, 27, 435.
- Sheldrick, G, M. “SHELXL97: Program for Crystal Structure Refinement”. Institut für Anorganische Chemie der Georg-August Universität Göttingen: Göttingen, Germany.
- Samples Availability: Available from the authors.


| O(1)-N(1) | 1.464(2) | C(3)-H(3B) | 1.02(3) | C(8)-H(8) | 0.93 | C(14)-H(14) | 0.93 |
| O(2)-C(2) | 1.194(2) | C(4)-H(4A) | 0.97(2) | C(9)-C(10) | 1.370(4) | C(15)-C(16) | 1.379(3) |
| O(3)-C(2) | 1.335(2) | C(4)-H(4B) | 0.97(2) | C(9)-H(9) | 0.93 | C(15)-H(15) | 0.93 |
| O(3)-C(3) | 1.462(3) | C(5)-C(6) | 1.544(2) | C(10)-C(11) | 1.377(4) | C(16)-C(17) | 1.376(3) |
| N(1)-C(13) | 1.420(2) | C(5)-H(5A) | 0.96(2) | C(10)-H(10) | 0.93 | C(16)-H(16) | 0.93 |
| N(1)-C(6) | 1.489(2) | C(5)-H(5B) | 0.95(2) | C(11)-C(12) | 1.385(3) | C(17)-C(18) | 1.385(3) |
| C(1)-C(5) | 1.513(2) | C(6)-C(7) | 1.518(2) | C(11)-H(11) | 0.93 | C(17)-H(17) | 0.93 |
| C(1)-C(4) | 1.513(2) | C(6)-H(6) | 0.94(2) | C(12)-H(12) | 0.93 | C(18)-H(18) | 0.93 |
| C(1)-C(2) | 1.535(2) | C(7)-C(8) | 1.383(2) | C(13)-C(18) | 1.391(2) | ||
| C(2)-O(3)-C(3) | 110.83(14) | C(1)-C(5)-C(6) | 104.52(14) | C(10)-C(11)-H(11) | 119.88(13) |
| C(13)-N(1)-O(1) | 108.46(11) | C(1)-C(5)-H(5A) | 112.4(13) | C(12)-C(11)-H(11) | 119.88(14) |
| C(13)-N(1)-C(6) | 118.77(12) | C(6)-C(5)-H(5A) | 113.0(12) | C(7)-C(12)-C(11) | 120.4(2) |
| O(1)-N(1)-C(6) | 105.18(11) | C(1)-C(5)-H(5B) | 109.6(12) | C(7)-C(12)-H(12) | 119.78(11) |
| O(1)-C(1)-C(5) | 103.40(13) | C(6)-C(5)-H(5B) | 108.3(13) | C(11)-C(12)-H(12) | 119.78(14) |
| O(1)-C(1)-C(4) | 111.98(14) | H(5A)-C(5)-H(5B) | 109(2) | C(18)-C(13)-C(14) | 119.1(2) |
| C(5)-C(1)-C(4) | 118.1(2) | N(1)-C(6)-C(7) | 110.89(13) | C(18)-C(13)-N(1) | 120.5(2) |
| O(1)-C(1)-C(2) | 104.04(12) | N(1)-C(6)-C(5) | 102.86(13) | C(14)-C(13)-N(1) | 120.12(14) |
| C(5)-C(1)-C(2) | 115.82(14) | C(7)-C(6)-C(5) | 114.07(14) | C(15)-C(14)-C(13) | 119.9(2) |
| C(4)-C(1)-C(2) | 102.92(13) | N(1)-C(6)-H(6) | 111.0(11) | C(15)-C(14)-H(14) | 120.03(12) |
| O(2)-C(2)-O(3) | 122.1(2) | C(7)-C(6)-H(6) | 108.7(11) | C(13)-C(14)-H(14) | 120.03(9) |
| O(2)-C(2)-C(1) | 127.6(2) | C(5)-C(6)-H(6) | 109.3(11) | C(16)-C(15)-C(14) | 120.8(2) |
| O(3)-C(2)-C(1) | 110.3(2) | C(8)-C(7)-C(12) | 118.9(2) | C(16)-C(15)-H(15) | 119.58(12) |
| O(3)-C(3)-C(4) | 105.7(2) | C(8)-C(7)-C(6) | 118.9(2) | C(14)-C(15)-H(15) | 119.58(11) |
| O(3)-C(3)-H(3A) | 105.8(14) | C(12)-C(7)-C(6) | 122.1(2) | C(17)-C(16)-C(15) | 119.3(2) |
| C(4)-C(3)-H(3A) | 115.1(14) | C(7)-C(8)-C(9) | 120.4(2) | C(17)-C(16)-H(16) | 120.34(11) |
| O(3)-C(3)-H(3B) | 104(2) | C(7)-C(8)-H(8) | 119.82(11) | C(15)-C(16)-H(16) | 120.34(12) |
| C(4)-C(3)-H(3B) | 111.5(14) | C(9)-C(8)-H(8) | 119.8(2) | C(16)-C(17)-C(18) | 120.7(2) |
| H(3A)-C(3)-H(3B) | 113(2) | C(10)-C(9)-C(8) | 120.3(2) | C(16)-C(17)-H(17) | 119.64(11) |
| C(3)-C(4)-C(1) | 104.4(2) | C(10)-C(9)-H(9) | 119.84(13) | C(18)-C(17)-H(17) | 119.64(12) |
| C(3)-C(4)-H(4A) | 111.6(12) | C(8)-C(9)-H(9) | 119.84(14) | C(17)-C(18)-C(13) | 120.1(2) |
| C(1)-C(4)-H(4A) | 109.2(12) | C(9)-C(10)-C(11) | 119.7(2) | C(17)-C(18)-H(18) | 119.95(12) |
| C(3)-C(4)-H(4B) | 112.6(13) | C(9)-C(10)-H(10) | 120.13(13) | C(13)-C(18)-H(18) | 119.95(10) |
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Cacciarini, M.; Cordero, F.M.; Faggi, C.; Goti, A. Cycloaddition Reactions of C,N-Diphenylnitrone to Methylene-γ-butyrolactones. Molecules 2000, 5, 637-647. https://doi.org/10.3390/50400637
Cacciarini M, Cordero FM, Faggi C, Goti A. Cycloaddition Reactions of C,N-Diphenylnitrone to Methylene-γ-butyrolactones. Molecules. 2000; 5(4):637-647. https://doi.org/10.3390/50400637
Chicago/Turabian StyleCacciarini, Martina, Franca M. Cordero, Cristina Faggi, and Andrea Goti. 2000. "Cycloaddition Reactions of C,N-Diphenylnitrone to Methylene-γ-butyrolactones" Molecules 5, no. 4: 637-647. https://doi.org/10.3390/50400637




