Teorethical Studies of the Stability of 8a-Alkyll-1,2,3,4,6,8ahexahydronaphtalen-1-ones Using Semiempirical Methods
Abstract
:Introduction
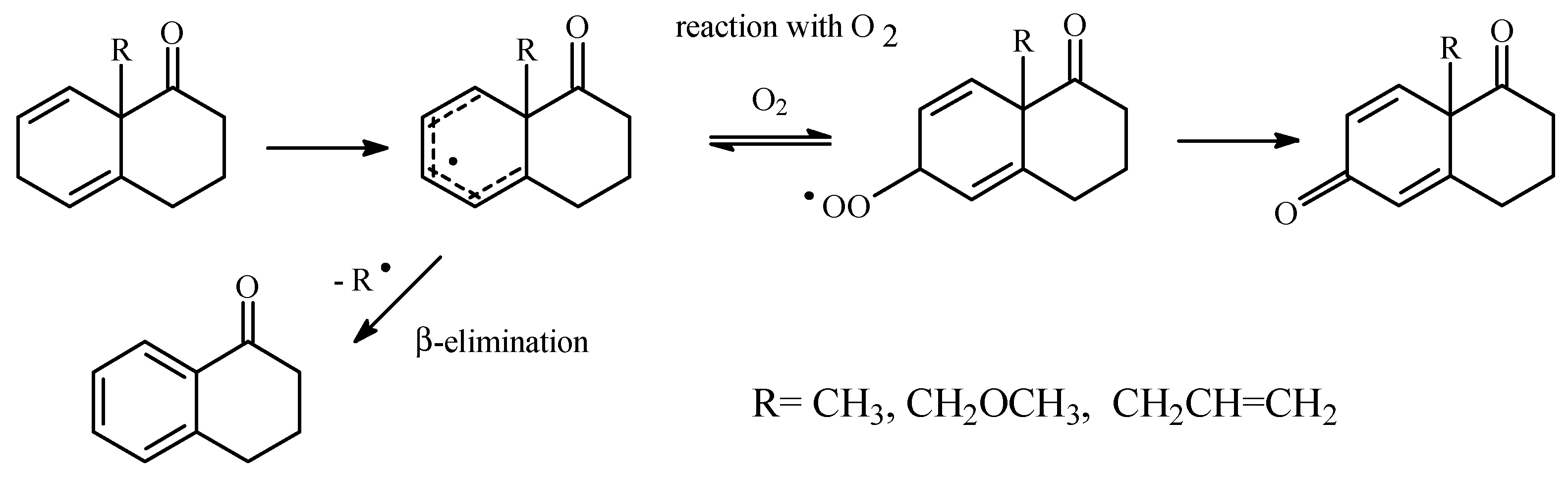
Results and Discussion
Acknowledgements
References and Notes
- (a) Vila, A. J.; Cravero, R. M.; González-Sierra, M. Tetrahedron Lett. 1991, 32, 1929–1932. (b) Vila, A. J.; Cravero, R. M.; González-Sierra, M. Tetrahedron 1993, 49, 4511–4526. (c) Labadie, G. R.; Cravero, R. M.; González-Sierra, M. Synth. Comm. 1996, 26, 4671–4684. (d) Labadie, G.R.; Cravero, R. M.; González-Sierra, M. submitted.
- Beckwith, A. L. J.; O´Shea, D. M.; Roberts, D. H. J. Am. Chem. Soc. 1986, 108, 6408–09.
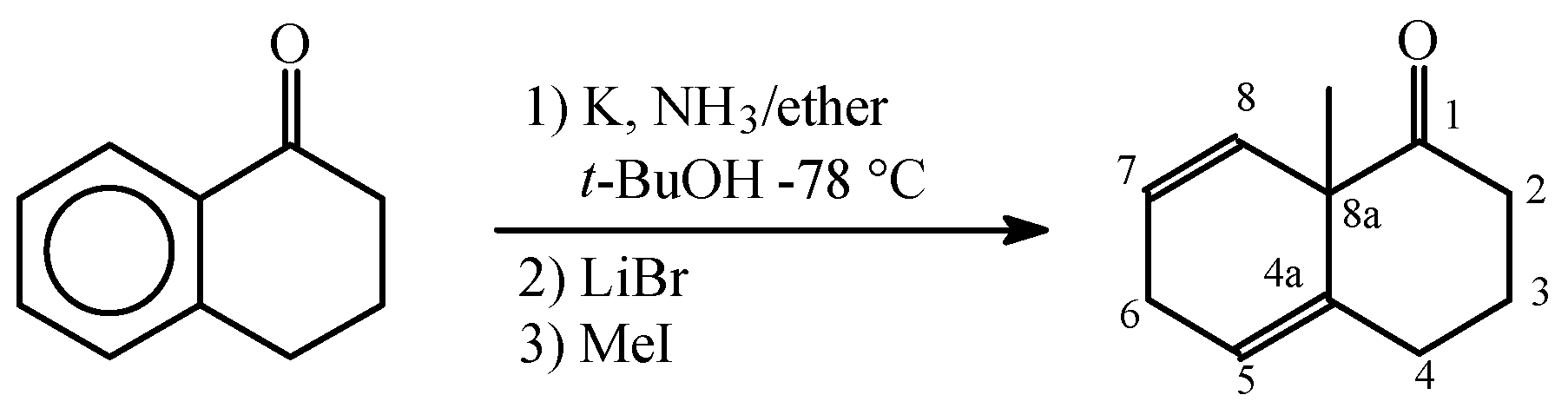
Share and Cite
Labadie, G.R.; Cravero, R.M.; Estiú, G.; Sierra, M.G. Teorethical Studies of the Stability of 8a-Alkyll-1,2,3,4,6,8ahexahydronaphtalen-1-ones Using Semiempirical Methods. Molecules 2000, 5, 453-454. https://doi.org/10.3390/50300453
Labadie GR, Cravero RM, Estiú G, Sierra MG. Teorethical Studies of the Stability of 8a-Alkyll-1,2,3,4,6,8ahexahydronaphtalen-1-ones Using Semiempirical Methods. Molecules. 2000; 5(3):453-454. https://doi.org/10.3390/50300453
Chicago/Turabian StyleLabadie, Guillermo R., Raquel M. Cravero, Guillermina Estiú, and Manuel Gonzalez Sierra. 2000. "Teorethical Studies of the Stability of 8a-Alkyll-1,2,3,4,6,8ahexahydronaphtalen-1-ones Using Semiempirical Methods" Molecules 5, no. 3: 453-454. https://doi.org/10.3390/50300453
APA StyleLabadie, G. R., Cravero, R. M., Estiú, G., & Sierra, M. G. (2000). Teorethical Studies of the Stability of 8a-Alkyll-1,2,3,4,6,8ahexahydronaphtalen-1-ones Using Semiempirical Methods. Molecules, 5(3), 453-454. https://doi.org/10.3390/50300453



