Photochemical Study of the Reactions of the 2-Naphtoxide Ion with Haloadamantanes
Abstract
:Introduction
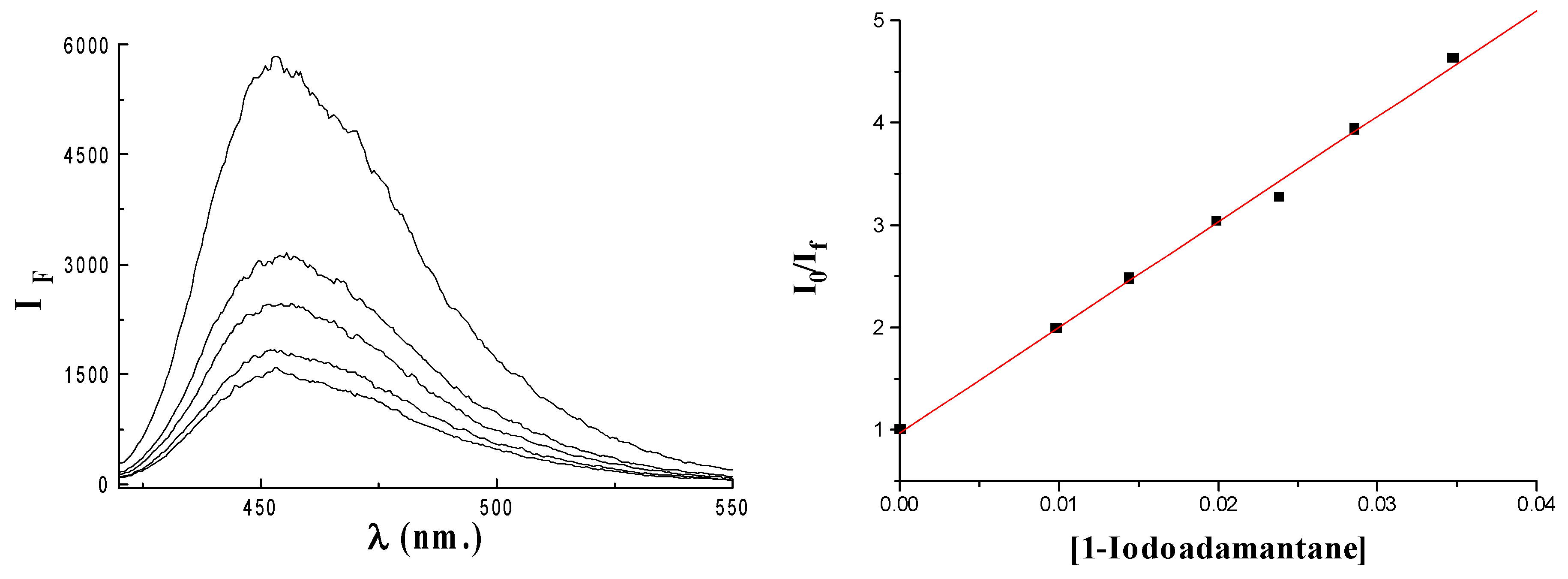
Results and Discussion
References and Notes
- Pierini, A. B.; Baumgartner, M. T.; Rossi, R. A. J. Org. Chem. 1991, 56, 580.
- Soumillion, J.; Vandereecken, P.; Van Der Auweraer, M.; De Schryver, F. C.; Schanck, A. J. Am. Chem. Soc. 1989, 111, 2217.
- Adcock, W.; Clark, C. I.; Houmam, A.; Krstic, A. R.; Pinson, J.; Savéant, J.-M.; Taylor, D. K.; Taylor, J. F. J. Am. Chem. Soc. 1994, 116, 4653.
- Murov, S. L.; Carmichael, I.; Hug, G. L. Handbook of Photochemistry, 2nd Edition; Marcel Dekker, Inc.: New York, 1993. [Google Scholar]
| X-Ada (Q) | kSV | kq (109 M-1s-1) | log kq | Ered [3] |
| 1-Iodoadamantane | 103 | 5.7 | 9.8 | -2,20 |
| 1-Bromoadamantane | 2.2 | 0.12 | 8.1 | -2,54 |
| 1-Chloroadamantane | 0.81 | 0.045 | 7.65 | -2,64 |
Share and Cite
Argüello, J.E.; Puiatti, M.; Peñéñory, A.B. Photochemical Study of the Reactions of the 2-Naphtoxide Ion with Haloadamantanes. Molecules 2000, 5, 455-456. https://doi.org/10.3390/50300455
Argüello JE, Puiatti M, Peñéñory AB. Photochemical Study of the Reactions of the 2-Naphtoxide Ion with Haloadamantanes. Molecules. 2000; 5(3):455-456. https://doi.org/10.3390/50300455
Chicago/Turabian StyleArgüello, Juan E., Marcelo Puiatti, and Alicia B. Peñéñory. 2000. "Photochemical Study of the Reactions of the 2-Naphtoxide Ion with Haloadamantanes" Molecules 5, no. 3: 455-456. https://doi.org/10.3390/50300455




