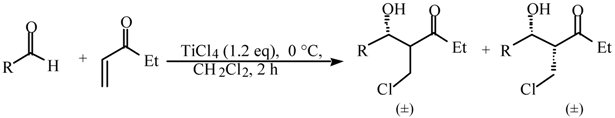
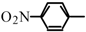
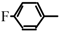
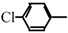
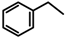
Halogeno Aldol Reaction of Ethyl Vinyl Ketone and Aldehydes Mediated by Titanium Tetrachloride
Abstract
: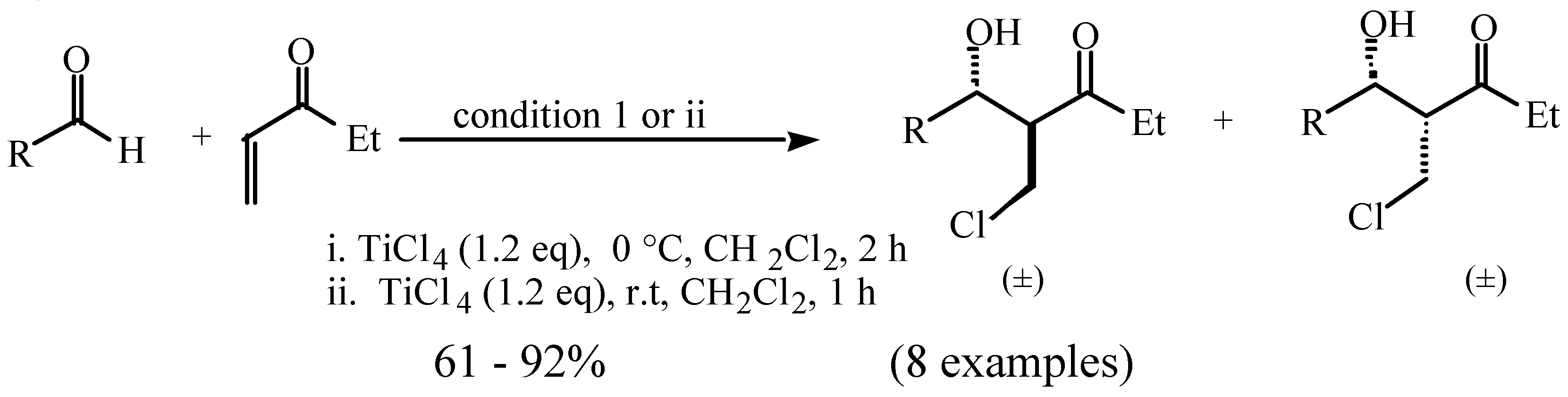
Introduction
Results and Discussion
 and
and  protons of aldol adducts [9]. For entry 1 of Table 1, the
protons of aldol adducts [9]. For entry 1 of Table 1, the  proton (CHOH) triplet of the anti isomer (d 5.11, J = 6.35 Hz) and the doublet-doublet of the anti isomer (d 5.05, J = 2.75, 6.08 Hz) were observed. The stability of these ethyl vinyl ketone-derived products and the resolution of their 1H-NMR spectra made this determination possible. In contrast, the methyl vinyl ketone-derived products can be very easily dehydrated under the current conditions. The syn selectivity suggests that this aldol reaction is dynamically controlled. This is similar to
proton (CHOH) triplet of the anti isomer (d 5.11, J = 6.35 Hz) and the doublet-doublet of the anti isomer (d 5.05, J = 2.75, 6.08 Hz) were observed. The stability of these ethyl vinyl ketone-derived products and the resolution of their 1H-NMR spectra made this determination possible. In contrast, the methyl vinyl ketone-derived products can be very easily dehydrated under the current conditions. The syn selectivity suggests that this aldol reaction is dynamically controlled. This is similar to  ,
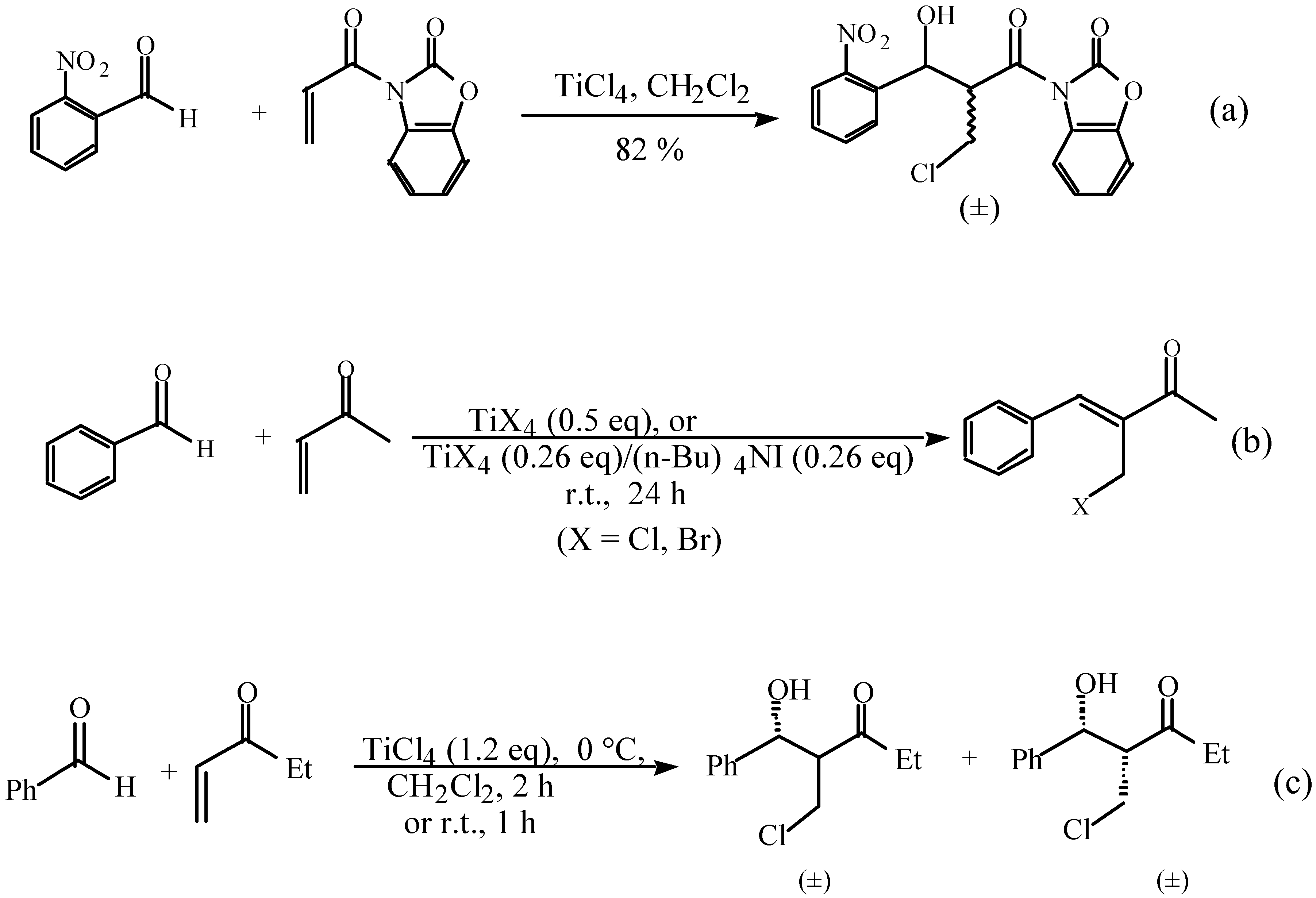
,  -unsaturated aldehyde-based system where the dynamically controlled syn stereoselection was proven to be dominant at -78 °C in the same solvent in which TiCl4/(n-Bu)4NI combination was employed as the halogen source (I-) [8c].
-unsaturated aldehyde-based system where the dynamically controlled syn stereoselection was proven to be dominant at -78 °C in the same solvent in which TiCl4/(n-Bu)4NI combination was employed as the halogen source (I-) [8c]. ,
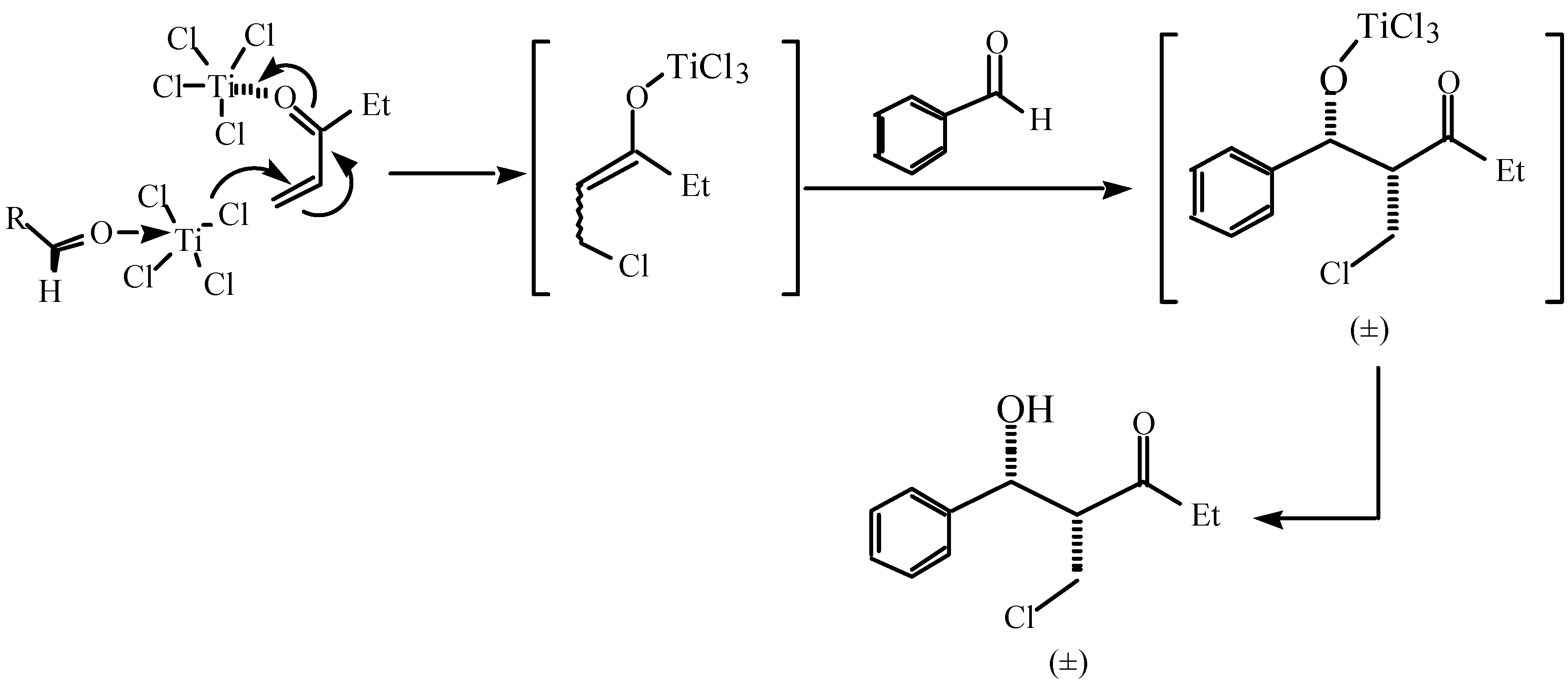
,  -conjugate double bond and to free the chlorine anion from TiCl4 prior to the
-conjugate double bond and to free the chlorine anion from TiCl4 prior to the  ,
,  -conjugate addition. The resulting Lewis acid species can also activate aldehyde for the subsequent aldol reaction by coordinating with aldehyde oxygen.
-conjugate addition. The resulting Lewis acid species can also activate aldehyde for the subsequent aldol reaction by coordinating with aldehyde oxygen.Conclusions
Experimental
General
Synthetic Method
Acknowledgments
References and Notes
- During the preparation of this manuscript, two halogeno aldol reaction papers have appeared in which the combinations of TiCl4 and Lewis bases were subjected to the Michael-type addition to methyl vinyl ketone followed by aldol reaction with aldehydes: (a) Kataoka, T.; Kinoshita, H.; Iwama, T.; Tsujiyama, S-i.; Iwamura, T.; Watanable, S-i.; Muraoka, O.; Tanabe, G. Tetrahedron 2000, 56, 4725. (b) Shi, M.; Jiang, J.-K.; Feng, Y.-S. Organic Letters 2000, 2, 2397.
- (a) Evans, D.A.; Nelson, J.V.; Taber, T.R. Topics in Stereochemistry 1982, 13, 1. (b) Heathcock, C.H. Comprehensive Carbonion Chemistry; Buncel, E., Durst, T., Eds.; Elsevier: New York, 1984; vol. 5B, p. 177. [Google Scholar] (c) Kim, B.M.; Williams, S.F.; Masumune, S. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Heathcock, C.H., Eds.; vol. 2, Chapter 1.7; Pergamon: Oxford, 1984; p. 239. [Google Scholar]
- For several recent reviews see: (a) Nelson, S.G. Tetrahedron Asymmetry 1998, 9, 357. (b) Mahrwald, R. Chem. Rev. 1999, 99, 1095. (c) Arya, P.; Qin, H. Tetrahedron 2000, 56, 917.
- For representative references on asymmetric catalytic Aldol reactions see: (a) Parmee, E.R.; Tempkin, O.; Masumune, S.; Abiko, A. J. Am. Chem. Soc. 1991, 113, 9365. (b) Corey, E.J.; Cywin, C.L.; Roper, T.D. Tetrahedron Lett. 1992, 33, 6907. (c) Evans, D.A.; Kozlowski, M.C.; Murray, J.A.; Burgey, C.S.; Campos, B.T.; Staples, R.J. J. Am. Chem. Soc. 1999, 121, 669. (d) Denmark, S.E.; Stavenger, R.A.; Wong, K.-T.; Su, X. J. Am. Chem. Soc. 1999, 121, 4982. (e) Taylor, S.; Duffey, M.O.; Morken, J.P. J. Am. Chem. Soc. 2000, 122, 4528.
- For recent reviews regarding the Baylis-Hillman reaction see: (a) Ciganek, E. Org. React. 1997, 51, 201. (b) Basavaiah, D.; Rao, P.D.; Hyma, R.S. Tetrahedron 1996, 52, 8001.
- Brzezinski, L.J.; Rafel, S.; Leahy, J.M. J. Am. Chem. Soc. 1997, 119, 4317. (b) Iwabuchi, Y.; Nakatani, M.; Yokoyama, N.; Hatakeyama, S. J. Am. Chem. Soc. 1999, 121, 10219. (c) Barrett, A.G.C.; Cook, A.S.; Kamimura, A. Chem. Commun. 1998, 2533. (d) Kawamura, M.; Kobayashi, S. Tetrahedron Lett. 1999, 40, 1539.
- (a) Li, G.; Wei, H.-X.; Caputo, T.D. Tetrahedron Lett. 2000, 41, 1. In this preliminary report, the mechanistic experiments of Baylis-Hillman system resulted in the halo aldol process as an extra synthetic bonus. The mixture of syn/anti isomers were generated, although the coupling contentsbased anti structural demonstration was temporally used. (b) Li, G.; Gao, J.; Wei, H.-X.; Enright, M. Organic Letters 2000, 2, 617.
- For TiCl4/(n-Bu)4NI combination see: (a) Taniguchi, M.; Hino, T.; Kishi, Y. Tetrahedron Lett. 1986, 39, 4767. (b) Yachi, K.; Maeda, K.; Shinokubo, H.; Oshima, K. Tetrahedron Lett. 1997, 38, 5161. (c) Uehira, S.; Han, Z.; Shinokubo, H.; Oshima, K. Organic Lett. 1999, 1, 1383.
- (a) Heng, K.; Smith, R.A.J. Tetrahedron 1979, 35, 425. (b) Kamimura, A.; Mitsudera, H.; Asano, S.; Kidera, S.; Kakehi, A. J. Org. Chem. 1999, 64, 6353.
- Wei, H.X.; Caputo, T.D.; Purkiss, D.W.; Li, G. Tetrahedron 2000, 56, 2397.
- For a comprehensive review about Lewis acid carbonyl complexation including TiCl4 see: Shambayati, S.; Schreiber, S.L. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; vol. 1, Pergamon: Oxford, 1991; pp. 283–321. [Google Scholar]
- Sample Availability: Available from the authors
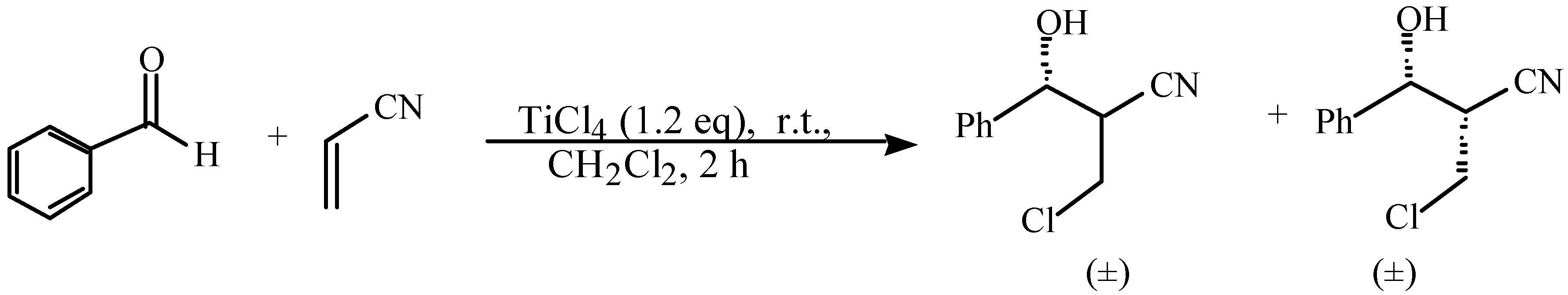
© 2000 MDPI. All rights reserved.
Share and Cite
Wei, H.-X.; Karur, S.; Li, G. Halogeno Aldol Reaction of Ethyl Vinyl Ketone and Aldehydes Mediated by Titanium Tetrachloride. Molecules 2000, 5, 1408-1416. https://doi.org/10.3390/51201408
Wei H-X, Karur S, Li G. Halogeno Aldol Reaction of Ethyl Vinyl Ketone and Aldehydes Mediated by Titanium Tetrachloride. Molecules. 2000; 5(12):1408-1416. https://doi.org/10.3390/51201408
Chicago/Turabian StyleWei, Han-Xun, Subramanian Karur, and Guigen Li. 2000. "Halogeno Aldol Reaction of Ethyl Vinyl Ketone and Aldehydes Mediated by Titanium Tetrachloride" Molecules 5, no. 12: 1408-1416. https://doi.org/10.3390/51201408