Application of Microwave in Organic Synthesis. Dry Synthesis of 2-Arylmethylene-3(2)-naphthofuranones
Abstract
:Introduction
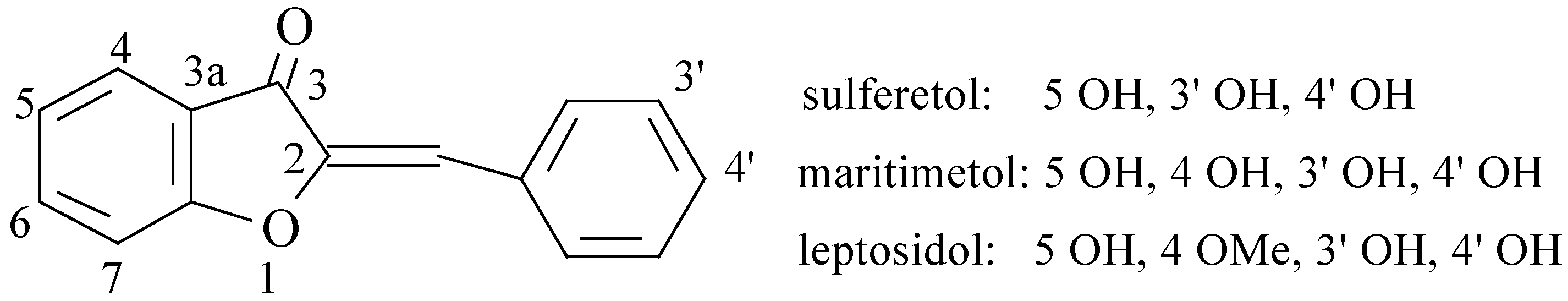


Results and Discussions
| δ (ppm) | C=O (a) | =CH (c) | C=(b) |
| aurones | 184.6 | 112.9 | 146.8 |
| flavones | 178.0 | 107.3 | 163.0 |
| product 4a | 184.7 | 113.1 | 147.6 |
| δ (ppm) | C=O (a) | =CH (c) | C=(b) |
| aurones (p-OMe) | 183-185 | 111-113 | 145-148 |
| flavones (o/p-OMe) | 177-178 | 106-112 | 160-163 |
| product 4d | 184.3 | 106.8 | 147.5 |
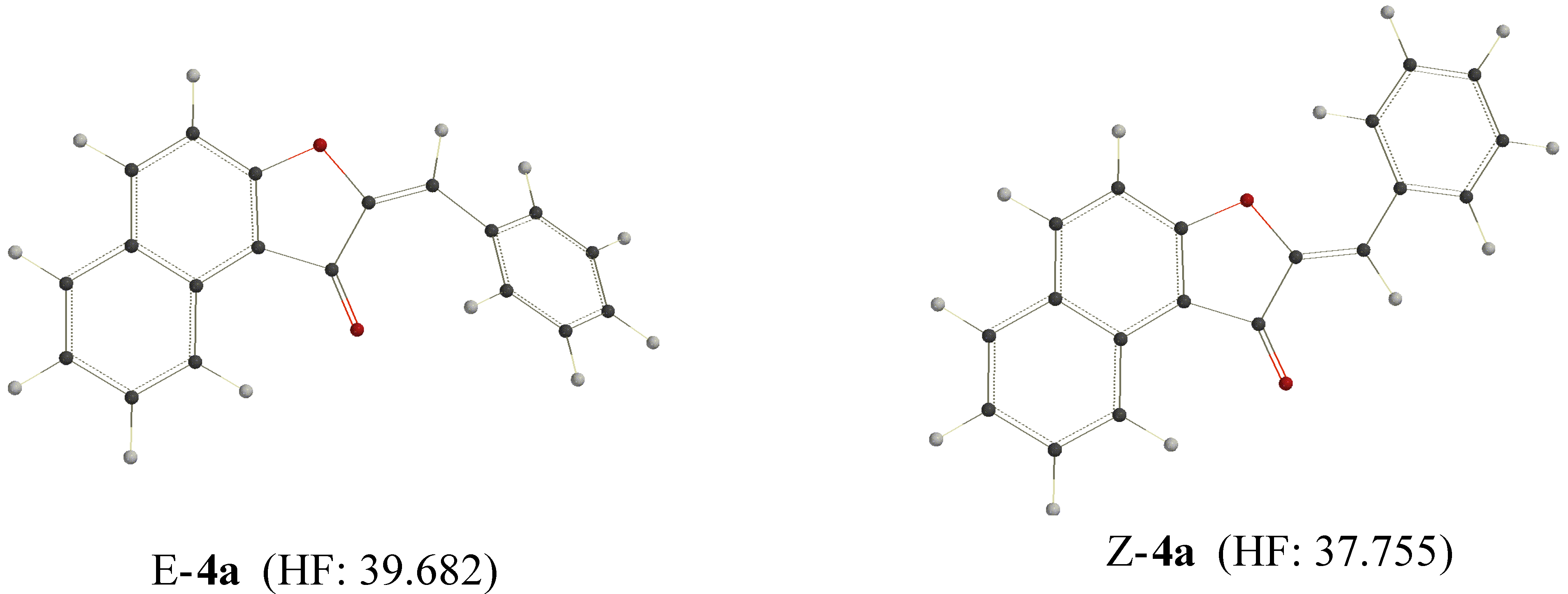
Conclusion
Experimental Section
General
Synthesis of 2H-naphtho[2,1-b]furan-1-one (1)
Synthesis of 2-(arylmethylene)-2H-naphtho[2,1-b]furan-1-one (5a-f)
General procedure
2-(Phenylmethylene)-2H-naphtho[2,1-b]furan-1-one (5a)
2-(4-Methoxyphenylmethylene)-2H-naphtho[2,1-b]furan- 1-one (5b)
2-(Fur-2-ylmethylene)-2H-naphtho[2,1-b]furan-1-one (5c)
2-(Thien-2-ylmethylene)-2H-naphtho[2,1-b]furan-1-one (5d)
2-(3,4-Dimethoxyphenylmethylene)-2H-naphtho[2,1-b]furan-1-one (5e)
2-(2,4,6-Trimethoxyphenylmethylene)-2H-naphtho[2,1-b]furan-1-one (5f)
References and Notes
- The Chemistry of Flavonoid Compounds; Geissman, T.A. (Ed.) Pergamon Press: London, 1962. ; The Flavonoids; Harbone, J.B.; Mabry, T.J.; Mabry, H. Chapman and Hal: London, 1975. [Google Scholar]
- Orzalesi, H.; Castel, J.; Flandre, O.; Darmanaden, R.; Damon, M. Ger. Offen. 2,829,619; Chem. Abstr.. 1979, 90, 197859z.
- Villemin, D.; Labiad, B. Synth. Commun. 1990, 20, 3207. Villemin, D.; Labiad, B. Synth. Commun. 1990, 20, 3213. Villemin, D.; Ricard, M. Synth. Commun. 1987, 17, 283. Villemin, D.; Ben Alloum, A.; Labiad, B. J. Chem. Soc. Chem. Commun. 1989, 386.
- Index des Produits Phytosanitaires, 20 ed.; ACTA: Paris, 1982. Villemin, D.; Hammadi, M. Synth. Commun. 1996, 26, 4337.
- Ullmann, G. Ber. 1897, 30, 1468.
- Varma, R. S.; Varma, M. Tetrahedron Lett. 1992, 33, 5937, and references cited.
- Villemin, D.; Martin, B. Synth. Commun. 1995, 25, 2319.
- Review on microwave activation. Bram, G.; Loupy, A.; Villemin, D. In Solid Supports and Catalysts in Organic Synthesis; chap.12; pp. 302–326. Caddick, S. Tetrahedron; 1995; Volume 51, pp. 10403–1043. Smith, K., Ed.; Ellis Horwood and Prentice Hall,1992. [Google Scholar] Langa, F.; de la Cruz, P.; de la Hoz, A.; Diaz-Ortiz, A.; Diez-Barra, E. Contemporary Organic Synthesis 1997, 373–386.
- Fitzgerald, D.M.; O'Sullivan, J.F.; Philbin, E.M.; Wheeler, T.S. J. Chem. Soc. 1955, 860.
- Donnelly, J.A.; Emerson, G.M. Tetrahedron 1990, 46, 7227. Donnelly, J.A.; Doran, H.J. Tetrahedron 1975, 31, 1565. Dean, F.M.; Podimuang, V. J. Chem. Soc. 1965, 3978.
- Pelter, A.; Ward, R.S.; Gray, T.I. J. Chem. Soc. Perkin I 1976, 2475. Pelter, A.; Ward, R.S.; Heller, H.G. J. Chem. Soc . Perkin I 1979, 328.
- HyperChem software from Hypercube Inc., Waterloo, Ontario, Canada
- Spartan software from Wavefunction Inc., 18401 Von Karman Avenue, Suite 370, Irvine, CA 92612, USA
- Ingham, B.H.; Stephen, H.; Timpe, R. J. Chem. Soc. 1931, 895.
- Sample Availability: available from the authors.
© 1998 MDPI. All rights reserved.
Share and Cite
Villemin, D.; Martin, B.; Bar, N. Application of Microwave in Organic Synthesis. Dry Synthesis of 2-Arylmethylene-3(2)-naphthofuranones. Molecules 1998, 3, 88-93. https://doi.org/10.3390/30300088
Villemin D, Martin B, Bar N. Application of Microwave in Organic Synthesis. Dry Synthesis of 2-Arylmethylene-3(2)-naphthofuranones. Molecules. 1998; 3(3):88-93. https://doi.org/10.3390/30300088
Chicago/Turabian StyleVillemin, Didier, Benoit Martin, and Nathalie Bar. 1998. "Application of Microwave in Organic Synthesis. Dry Synthesis of 2-Arylmethylene-3(2)-naphthofuranones" Molecules 3, no. 3: 88-93. https://doi.org/10.3390/30300088
APA StyleVillemin, D., Martin, B., & Bar, N. (1998). Application of Microwave in Organic Synthesis. Dry Synthesis of 2-Arylmethylene-3(2)-naphthofuranones. Molecules, 3(3), 88-93. https://doi.org/10.3390/30300088




