A Three Step Synthesis of 11-Cycloheptylundecanoic Acid, a Component of the Thermoacidophile Alicyclobacillus cycloheptanicus
Abstract
:Introduction
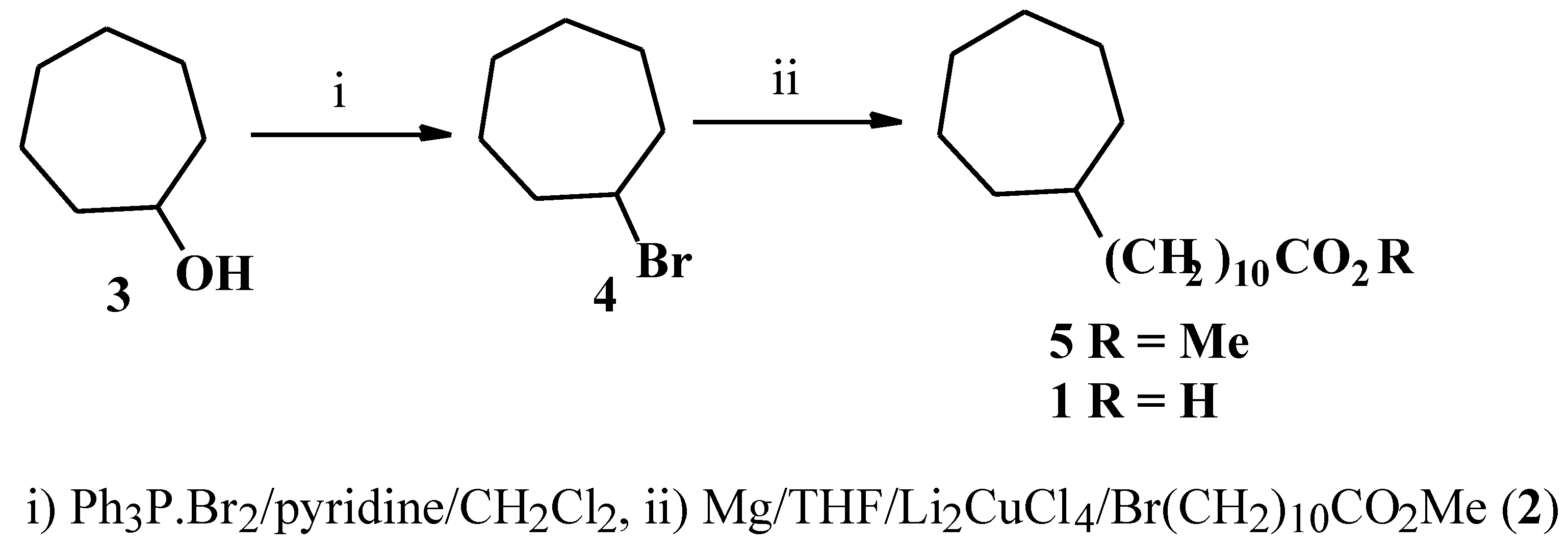
Results and Discussion
Experimental
General
Cycloheptyl bromide (4)
Methyl 11-Cycloheptylundecanoate (5)
References
- Dienhard, G.; Sarr, J.; Krischke, W.; Poralla, K. Syst. Appl. Microbiol. 1987, 10, 68–73.
- Allgaier, H.; Poralla, K.; Jung, G. Liebigs Ann. Chem. 1985, 378–382.
- Kannenberg, E.; Blume, A.; Poralla, K. Biochim. Biophys. Acta 1984, 172, 331–334.
- Moore, B. S.; Floss, H. G.; Poralla, K. J. Nat. Prod. 1995, 58, 590–593. [CrossRef]
- Chattopadhyay, A. Ph. D. Thesis, The University of Bombay, Bombay, India, 1989.
- Wiley, G. A.; Hershkowitz, R. I.; Rein, B. M.; Chung, B. C. J. Am. Chem. Soc. 1964, 86, 964–965. [CrossRef]
- Sample Availability: Samples available from the authors.
© 1998 MDPI. All rights reserved. Molecules http://www.mdpi.org/molecules/
Share and Cite
Hassarajani, S.A.; Mamdapur, V.R. A Three Step Synthesis of 11-Cycloheptylundecanoic Acid, a Component of the Thermoacidophile Alicyclobacillus cycloheptanicus. Molecules 1998, 3, 41-43. https://doi.org/10.3390/30200041
Hassarajani SA, Mamdapur VR. A Three Step Synthesis of 11-Cycloheptylundecanoic Acid, a Component of the Thermoacidophile Alicyclobacillus cycloheptanicus. Molecules. 1998; 3(2):41-43. https://doi.org/10.3390/30200041
Chicago/Turabian StyleHassarajani, Sham A., and Vasant R. Mamdapur. 1998. "A Three Step Synthesis of 11-Cycloheptylundecanoic Acid, a Component of the Thermoacidophile Alicyclobacillus cycloheptanicus" Molecules 3, no. 2: 41-43. https://doi.org/10.3390/30200041



