Luminescent 1,10-Phenanthroline β-Diketonate Europium Complexes with Large Second-Order Nonlinear Optical Properties
Abstract
:1. Introduction
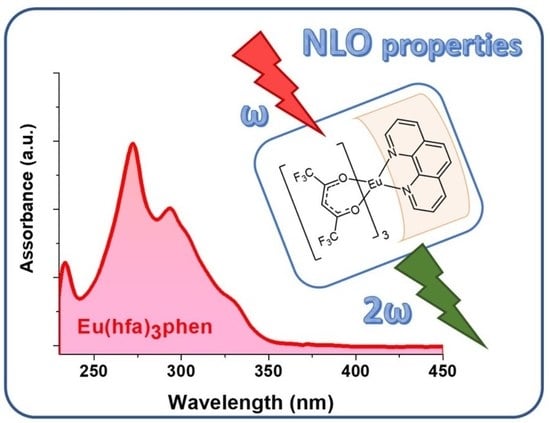
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Prasad, N.P.; Williams, D.J. Introduction to Nonlinear Optical Effects in molecules and Polymers; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Lupo, D. Molecular Nonlinear Optics: Materials, Physics and Devices; Academic Press: Boston, MA, USA, 1994. [Google Scholar]
- Kanis, D.R.; Ratner, M.A.; Marks, T.J. Design and Construction of Molecular Assemblies with Large Second-Order Optical Nonlinearities. Quantum Chemical Aspects. Chem. Rev. 1994, 94, 195–242. [Google Scholar] [CrossRef]
- Beverina, L.; Ruffo, R.; Patriarca, G.; De Angelis, F.; Roberto, D.; Righetto, S.; Ugo, R.; Pagani, G. Second harmonic generation in nonsymmetrical squaraines: Tuning of the directional charge transfer character in highly delocalized dyes. J. Mater. Chem. 2009, 19, 8190–8197. [Google Scholar] [CrossRef]
- Todescato, F.; Fortunati, I.; Carlotto, S.; Ferrante, C.; Grisanti, L.; Sissa, C.; Painelli, A.; Colombo, A.; Dragonetti, C.; Roberto, D. Dimers of polar chromophores in solution: Role of excitonic interactions in one- and two-photon absorption properties. Phys. Chem. Chem. Phys. 2011, 13, 11099–11109. [Google Scholar] [CrossRef]
- Marinotto, D.; Castagna, R.; Righetto, S.; Dragonetti, C.; Colombo, A.; Bertarelli, C.; Garbugli, M.; Lanzani, G. Photoswitching of the Second Harmonic Generation from Poled Phenyl-Substituted Dithienylethenes Thin Film and EFISH Measurements. J. Phys. Chem. C 2011, 115, 20425–20432. [Google Scholar] [CrossRef]
- Powel, C.E.; Humphrey, M.G. Nonlinear otical properties of transition metal acetylides and their derivatives. Coord. Chem. Rev. 2004, 248, 725–756. [Google Scholar] [CrossRef]
- Di Bella, S. Second-order nonlinear optical properties of transition metal complexes. Chem. Soc. Rev. 2001, 30, 355–366. [Google Scholar] [CrossRef]
- Coe, B.J. Nonlinear Optical Properties of Metal Complexes. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier Pergamon: Oxford, UK, 2004; Volume 9, pp. 621–687. [Google Scholar]
- Maury, O.; Le Bozec, H. Molecular Engineering of Octupolar NLO Molecules and Materials Based on Bipyridyl Metal Complexes. Acc. Chem. Res. 2005, 38, 691–704. [Google Scholar] [CrossRef]
- Cariati, E.; Pizzotti, M.; Roberto, D.; Tessore, F.; Ugo, R. Coordination and organometallic compounds and inorganic–organic hybrid crystalline materials for second-order non-linear optics. Coord. Chem. Rev. 2006, 250, 1210–1233. [Google Scholar] [CrossRef]
- Morrall, J.P.; Dalton, G.T.; Humphrey, M.G.; Samoc, M. Organotransition metal complexes for nonlinear optics. Adv. Organomet. Chem. 2007, 55, 61–136. [Google Scholar] [CrossRef]
- Di Bella, S.; Dragonetti, C.; Pizzotti, M.; Roberto, D.; Tessore, F.; Ugo, R. Molecular Organometallic Materials for Optics. In Topics in Organometallic Chemistry 28, Le Bozec, H., Guerchais, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Maury, O.; Le Bozec, H. Metal-based quadratic molecular materials. In Molecular Materials; Bruce, D.W., O’Hare, D., Walton, R.I., Eds.; Wiley: Chichester, UK, 2010. [Google Scholar]
- Hannachi, D.; Fahim Haroun, M.; Khireddine, A.; Chermette, H. Optical and nonlinear optical properties of Ln(Tp)2, where Ln = La,...,Lu and Tp = tris(pyrazolyl)borate: A DFT+TD-DFT study. New J Chem. 2019, 43, 14377–14389. [Google Scholar] [CrossRef]
- Rossi, E.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D.; Ugo, R.; Valore, A.; Williams, J.A.G.; Lobello, M.G.; De Angelis, F.; et al. Tuning the dipolar second-order nonlinear optical properties of cyclometallated platinum(II) complexes with tridentate N^C^N-binding ligands. Chem. Eur. J. 2013, 19, 9875–9883. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Dragonetti, C.; Marinotto, D.; Righetto, S.; Roberto, D.; Tavazzi, S.; Escadeillas, M.; Guerchais, V.; Le Bozec, H.; Boucekkine, A.; et al. Cyclometalated 4-styryl-2-phenylpyridine Pt(II) acetylacetonate complexes as second-order NLO building blocks for SHG active polymeric films. Organometalllics 2013, 32, 3890–3894. [Google Scholar] [CrossRef]
- Colombo, A.; Nisic, F.; Dragonetti, C.; Marinotto, D.; Oliveri, I.P.; Righetto, S.; Lobello, M.G.; De Angelis, F. Unexpectedly high second-order nonlinear optical properties of simple Ru and Pt alkynyl complexes as an analytical springboard for NLO-active polymer films. Chem. Commun. 2014, 50, 7986–7989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabu, S.; David, E.; Viswanathan, T.; Thirumoorthy, K.; Panda, T.; Dragonetti, C.; Colombo, A.; Marinotto, D.; Righetto, S.; Roberto, D.; et al. NLO-active Y-shaped ferrocene conjugated imidazole chromophores as precursors for SHG polymeric films. Dalton Trans. 2020, 49, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Dragonetti, C.; Guerchais, V.; Hierlinger, H.; Zysman-Colman, E.; Roberto, D. A trip in the nonlinear optical properties of iridium complexes. Coord. Chem. Rev. 2020, 414, 213293. [Google Scholar] [CrossRef]
- David, E.; Colombo, A.; Dragonetti, C.; Palanisami, N. Novel Ferrocene-Appended β-Ketoimines and Related BF2 Derivatives with Significant Aggregation-Induced Emission and Second-Order Nonlinear Optical Properties. Eur. J. Chem. 2021, 27, 7124–7137. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Guerchais, V.; Roberto, D. An excursion in the second-order nonlinear optical properties of platinum complexes. Coord. Chem. Rev. 2021, 446, 214113. [Google Scholar] [CrossRef]
- Aubert, V.; Guerchais, V.; Ishow, E.; Hoang-Thy, K.; Ledoux, I.; Nakatani, K.; Le Bozec, H. Efficient Photoswitching of the Nonlinear Optical Properties of Dipolar Photochromic Zinc(II) Complexes. Angew. Chem. Int. Ed. 2008, 47, 577–580. [Google Scholar] [CrossRef] [Green Version]
- Dragonetti, C.; Righetto, S.; Roberto, D.; Ugo, R.; Valore, A.; Demartin, F.; De Angelis, F.; Sgamellotti, A.; Fantacci, S. The role of 5-R-1,10-phenanthroline (R = CH3, NO2) on the emission properties and second-order NLO response of cationic Ir(III) organometallic chromophores. Inorg. Chim. Acta 2008, 361, 4070–4076. [Google Scholar] [CrossRef]
- Dragonetti, C.; Righetto, S.; Roberto, D.; Ugo, R.; Valore, A.; Fantacci, A.; Sgamellotti, A.; De Angelis, F. Cyclometallated Iridium(III) complexes with substituted 1,10-phenanthrolines: A new class of highly active organometallic second order NLO-phores with excellent transparency with respect to second harmonic emission. Chem. Commun. 2007, 40, 4116–4118. [Google Scholar] [CrossRef]
- Hierlinger, C.; Bradford Cordes, D.; Slawin, A.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D.; Jacquemin, D.; Zysman-Colman, E.; Guerchais, V. An investigation on the second-order nonlinear optical response of cationic bipyridine or phenanthroline iridium(III) complexes bearing cyclometallated 2-phenylpyridines with a triphenylamine substituent. Dalton Trans. 2018, 47, 8292–8300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, R.; Jaque, D. Doping Lanthanide Ions in Colloidal Semiconductor Nanocrystals for Brighter Photoluminescence. Chem. Rev. 2021, 121, 1425. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, G.; Bettinelli, M.; Speghini, A.; Fragalà, I.L. Europium “second generation” precursors for metal-organic chemical vapor deposition: Characterization and optical spectroscopy. Eur. J. Inorg. Chem. 2001, 2001, 1039–1044. [Google Scholar] [CrossRef]
- Picot, A.; D’Aléo, A.; Baldeck, P.L.; Grichine, A.; Duperray, A.; Andraud, C.; Maury, O. Long-Lived Two-Photon Excited Luminescence of Water-Soluble Europium Complex: Applications in Biological Imaging Using Two-Photon Scanning Microscopy. J. Am. Chem. Soc. 2008, 130, 1532–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walton, J.W.; Bourdolle, A.; Butler, S.J.; Soulie, M.; Delbianco, M.; McMahon, B.K.; Pal, R.; Puschmann, H.; Zwier, J.M.; Lamarque, L.; et al. Very bright europium complexes that stain cellular mitochondria. Chem. Commun. 2013, 49, 1600–1602. [Google Scholar] [CrossRef] [Green Version]
- Hamon, N.; Galland, M.; Le Fur, M.; Roux, A.; Duperray, A.; Grichine, A.; Andraud, C.; Le Guennic, B.; Beyler, M.; Maury, O.; et al. Combining a pyclen framework with conjugated antenna for the design of europium and samarium luminescent bioprobes. Chem. Commun. 2018, 54, 6173–6176. [Google Scholar] [CrossRef]
- Hamon, N.; Roux, A.; Beyler, M.; Mulatier, J.-C.; Andraud, C.; Nguyen, C.; Maynadier, M.; Bettache, N.; Duperray, A.; Grichine, A.; et al. Pyclen-Based Ln(III) Complexes as Highly Luminescent Bioprobes for In Vitro and In Vivo One- and Two-Photon Bioimaging Applications. J. Am. Chem. Soc. 2020, 142, 10184–10197. [Google Scholar] [CrossRef]
- Galán, L.A.; Hamon, N.; Nguyen, C.; Molnár, E.; Kiss, J.; Mendy, J.; Hadj-Kaddour, K.; Onofre, M.; Trencsényi, G.; Monnereau, C.; et al. Design of polyazamacrocyclic Gd3+ theranostic agents combining magnetic resonance imaging and two-photon photodynamic therapy. Inorg. Chem. Front. 2021, 8, 2213–2224. [Google Scholar] [CrossRef]
- Sénéchal, K.; Toupet, L.; Ledoux, I.; Zyss, J.; Le Bozec, H.; Maury, O. First lanthanide dipolar complexes for second-order nonlinear optics. Chem. Commun. 2004, 19, 2180–2181. [Google Scholar] [CrossRef]
- Tancrez, N.; Feuvrie, C.; Ledoux, I.; Zyss, J.; Toupet, L.; Le Bozec, H.; Maury, O. Lanthanide Complexes for Second Order Nonlinear Optics: Evidence for the Direct Contribution of f Electrons to the Quadratic Hyperpolarizability. J. Am. Chem. Soc. 2005, 127, 13474–13475. [Google Scholar] [CrossRef]
- Sénéchal-David, K.; Hemeryck, A.; Tancrez, N.; Toupet, L.; Williams, J.A.G.; Ledoux, I.; Zyss, J.; Boucekkine, A.; Guégan, J.-P.; Le Bozec, H.; et al. Synthesis, Structural Studies, Theoretical Calculations, and Linear and Nonlinear Optical Properties of Terpyridyl Lanthanide Complexes: New Evidence for the Contribution of f Electrons to the NLO Activity. J. Am. Chem. Soc. 2006, 128, 12243–12255. [Google Scholar] [CrossRef] [PubMed]
- Andraud, C.; Maury, O. Lanthanide Complexes for Nonlinear Optics: From Fundamental Aspects to Applications. Eur. J. Inorg. Chem. 2009, 2009, 4357–4371. [Google Scholar] [CrossRef]
- Valore, A.; Cariati, E.; Righetto, S.; Roberto, D.; Tessore, F.; Ugo, R.; Fragalà, I.L.; Fragalà, M.E.; Malandrino, G.; De Angelis, F.; et al. Fluorinated β-Diketonates Diglyme Lanthanide Complexes as New Second Order NLO Chromophores: The Role of f Electrons on the Dipolar and Octupolar Contribution to the Quadratic Hyperpolarizability. J. Am. Chem. Soc. 2010, 132, 4966–4970. [Google Scholar] [CrossRef]
- Gulino, A.; Fragalà, I.L.; Lupo, F.; Malandrino, G.; Motta, A.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D.; Ugo, R.; et al. Fascinating Role of the Number of f Electrons in Dipolar and Octupolar Contributions to Quadratic Hyperpolarizability of trinuclear lanthanides-biscopper Schiff base complexes. Inorg. Chem. 2013, 52, 7550–7556. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, R.; Dutta, A.; Roy, S.; Das, G.; Ledoux-Rak, I.; Mondal, P.; Prasad, S.K.; Rao, D.S.S.; Bhattacharjee, C.R. Multifunctional Lanthanide Complexes: Mesomorphism, Photoluminescence and Second Order NLO Property. Chem. Sel. 2018, 3, 8245–8251. [Google Scholar] [CrossRef]
- Furet, E.; Costuas, K.; Rabiller, P.; Maury, O. On the Sensitivity of f Electrons to Their Chemical Environment. J. Am. Chem. Soc. 2008, 130, 2180–2183. [Google Scholar] [CrossRef]
- Law, G.-L.; Wong, K.-L.; Lau, K.-K.; Lap, S.-T.; Tanner, P.A.; Kuo, F.; Wong, W.-T. Nonlinear optical activity in dipolar organic–lanthanide complexes. J. Mater. Chem. 2010, 20, 4074–4079. [Google Scholar] [CrossRef]
- Walton, J.W.; Carr, R.; Evans, N.H.; Funk, A.M.; Kenwright, A.M.; Parker, D.; Yufit, D.S.; Botta, M.; De Pinto, S.; Wong, K.-L. Isostructural series of nine-coordinate chiral lanthanide complexes based on triazacyclononane. Inorg. Chem. 2012, 51, 8042–8056. [Google Scholar] [CrossRef]
- Maker, P.D. Spectral broadening of elastic second-harmonic light scattering in liquids. Phys. Rev. A 1970, 1, 923–951. [Google Scholar] [CrossRef]
- Clays, K.; Persoons, A. Hyper-Rayleigh scattering in solution. Phys. Rev. Lett. 1991, 66, 2980–2983. [Google Scholar] [CrossRef]
- Zyss, J. Molecular engineering implications of rotational invariance in quadratic nonlinear optics: From dipolar to octupolar molecules and materials. J. Chem. Phys. 1993, 98, 6583–6600. [Google Scholar] [CrossRef]
- Zyss, J.; Ledoux, I. Nonlinear optics in multipolar media: Theory and experiments, Chem. Rev. 1994, 94, 77–105. [Google Scholar] [CrossRef]
- Ledoux, I.; Zyss, J. Influence of the molecular environment in solution measurements of the second-order optical susceptibility for urea and derivatives. Chem. Phys. 1982, 73, 203–213. [Google Scholar] [CrossRef]
- Bunzli, J.-C.G.; Pecharsky, V.K. Handbook on the Physics and Chemistry of Rare Earths: Including Actinides; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Singer, K.D.; Sohn, J.E.; King, L.A.; Gordon, H.M.; Katz, H.E.; Dirk, C.W. Second-order nonlinear-optical properties of donor- and acceptor-substituted aromatic compounds. J. Opt. Soc. Am. B 1989, 6, 1339–1350. [Google Scholar] [CrossRef]
- Dirk, C.W.; Katz, H.E.; Schilling, M.L.; King, L.A. Use of thiazole rings to enhance molecular second-order nonlinear optical susceptibilities. Chem. Mater. 1990, 2, 700–705. [Google Scholar] [CrossRef]
- Roberto, D.; Ugo, R.; Tessore, F.; Lucenti, E.; Quici, S.; Vezza, S.; Fantucci, P.C.; Invernizzi, I.; Bruni, S.; Ledoux-Rak, I.; et al. Effect of the Coordination to M(II) Metal Centers (M = Zn, Cd, Pt) on the Quadratic Hyperpolarizability of Various Substituted 5-X-1,10-phenanthrolines (X = Donor Group) and of trans-4-(Dimethylamino)-4′-stilbazole. Organometallics 2002, 21, 161–170. [Google Scholar] [CrossRef]
- Di Bella, S.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D. Zinc(II) as a versatile template for efficient dipolar and octupolar second-order nonlinear optical molecular materials. Inorganics 2018, 6, 133. [Google Scholar] [CrossRef] [Green Version]
- Strasser, A.; Vogler, A. Optical properties of thallium(I), lead(II) and bismuth(III) hexafluoroacetylacetonates. Intraligand phosphorescence under ambient conditions. Inorg. Chem. Commun. 2004, 7, 528–530. [Google Scholar] [CrossRef]
- Strasser, A.; Vogler, A. Intraligand phosphorescence of lead(II) β-diketonates under ambient conditions. J. Photobiochem. Photobiol. A Chem. 2004, 165, 115–118. [Google Scholar] [CrossRef]
- Shurygin, A.V.; Korochentsev, V.V.; Cherednichenko, A.I.; Mirochnik, A.G.; Kalinovskaya, I.V.; Vovna, V.I. Electronic structure and optical properties of Eu(III) tris-β-diketonate adducts with 1,10-phenanthroline. J. Mol. Struct. 2018, 1155, 133–142. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 195, 1–45. [Google Scholar] [CrossRef]




| Compound | Absorption a λmax/nm (ε/M−1 cm−1) | μβ (×10−48 esu) b |
|---|---|---|
1 | 233, 272 (11,851), 293 | 1016 |
2 | 230, 272 (9347), 341 (10,000) | 920 |
3 | 306 (16,617) | 161 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagnani, F.; Colombo, A.; Malandrino, G.; Dragonetti, C.; Pellegrino, A.L. Luminescent 1,10-Phenanthroline β-Diketonate Europium Complexes with Large Second-Order Nonlinear Optical Properties. Molecules 2022, 27, 6990. https://doi.org/10.3390/molecules27206990
Fagnani F, Colombo A, Malandrino G, Dragonetti C, Pellegrino AL. Luminescent 1,10-Phenanthroline β-Diketonate Europium Complexes with Large Second-Order Nonlinear Optical Properties. Molecules. 2022; 27(20):6990. https://doi.org/10.3390/molecules27206990
Chicago/Turabian StyleFagnani, Francesco, Alessia Colombo, Graziella Malandrino, Claudia Dragonetti, and Anna Lucia Pellegrino. 2022. "Luminescent 1,10-Phenanthroline β-Diketonate Europium Complexes with Large Second-Order Nonlinear Optical Properties" Molecules 27, no. 20: 6990. https://doi.org/10.3390/molecules27206990