Pharmacokinetic Interactions between Canagliflozin and Sorafenib or Lenvatinib in Rats
Abstract
:1. Introduction
2. Results
2.1. Method Development and Optimization
2.2. Method Validation
2.3. Pharmacokinetic Interactions between Sorafenib and Canagliflozin
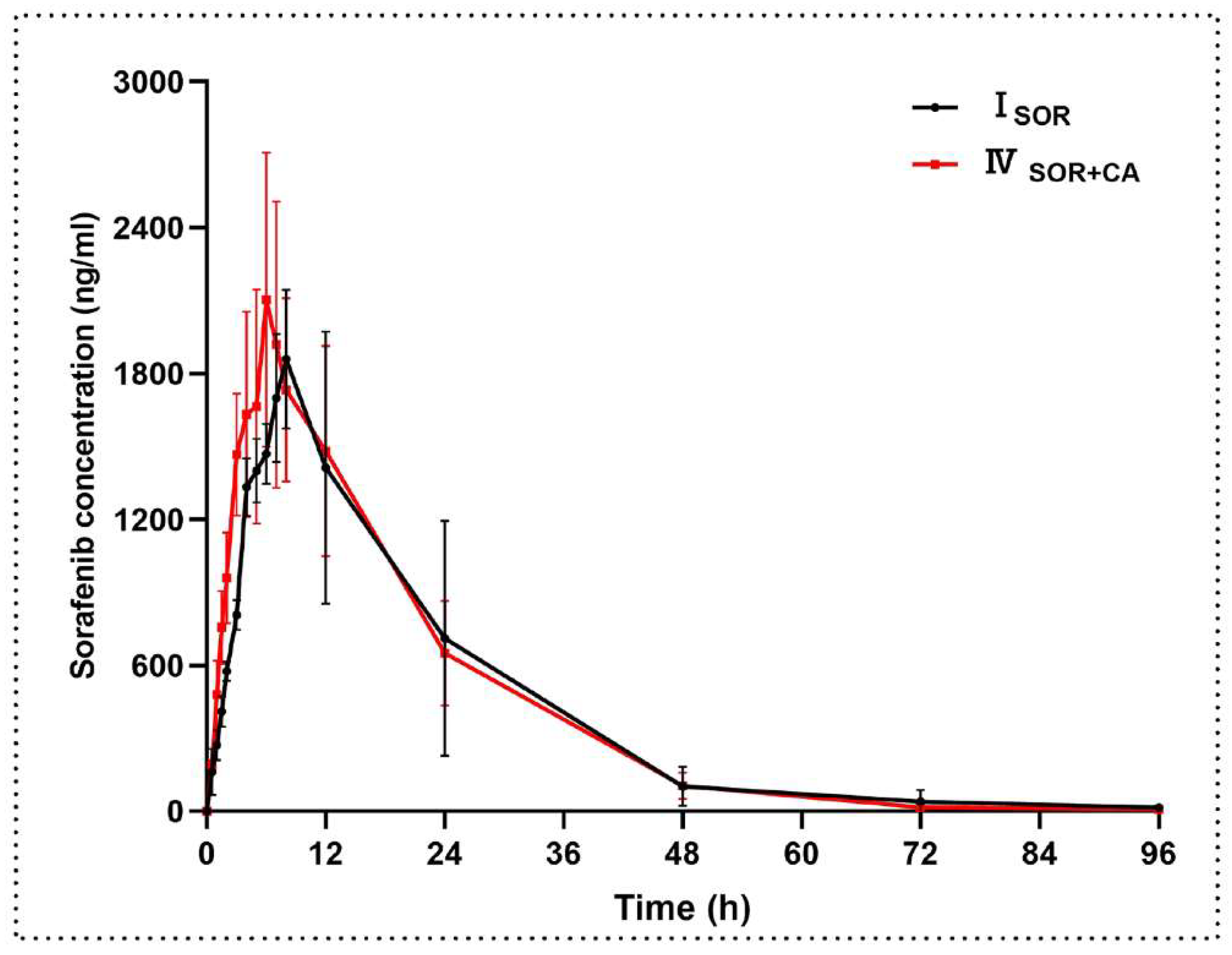
2.3.1. The Effect of Canagliflozin on the Pharmacokinetics of Sorafenib
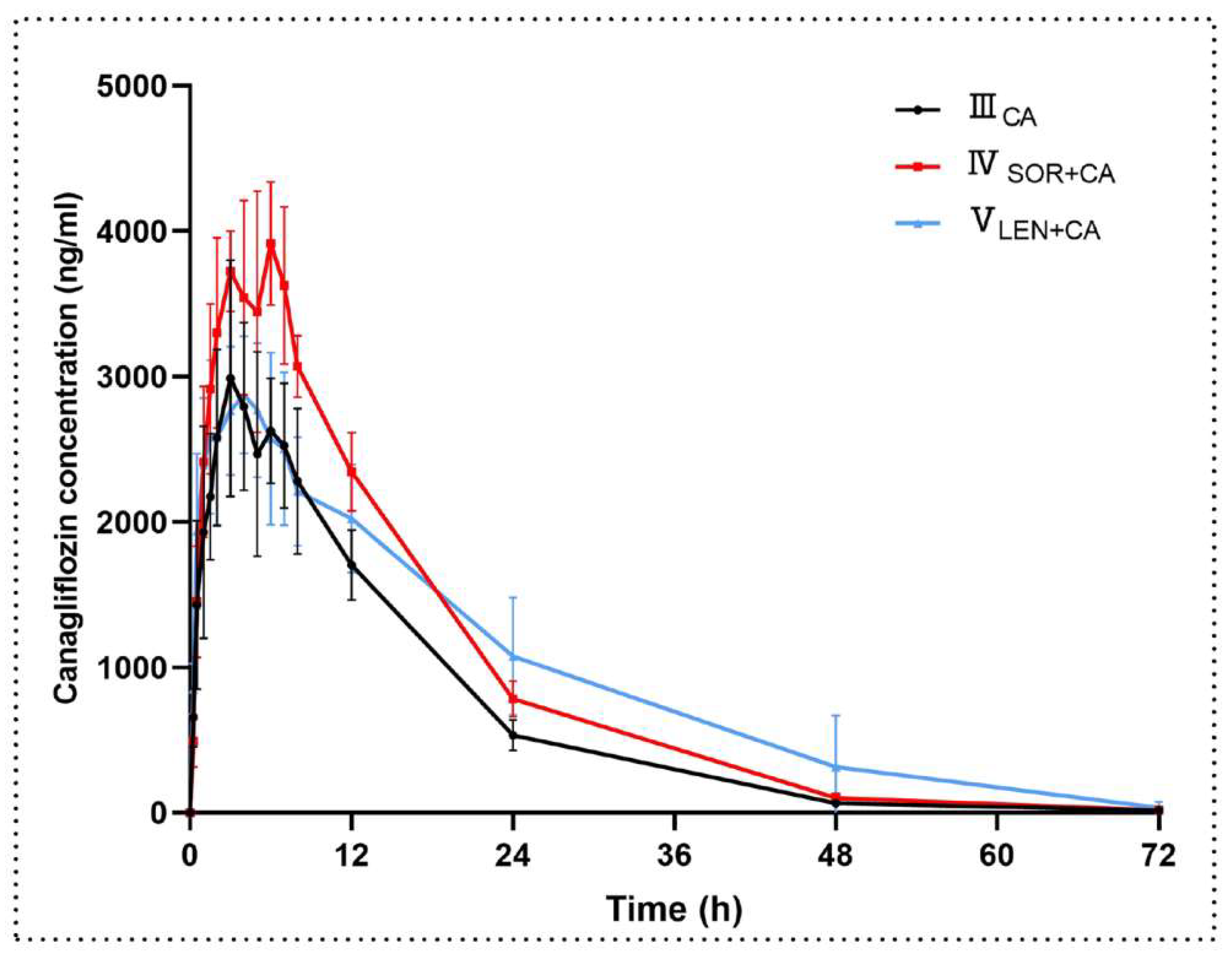
2.3.2. The Effect of Sorafenib on the Pharmacokinetics of Canagliflozin
2.4. Pharmacokinetic Interactions between Lenvatinib and Canagliflozin
2.4.1. The Effect of Canagliflozin on the Pharmacokinetics of Lenvatinib
2.4.2. The Effect of Lenvatinib on the Pharmacokinetics of Canagliflozin
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Preparation of Calibration Standards and Quality Control (QC) Samples
4.4. Plasma Sample Preparation
4.5. UPLC–MS/MS Conditions
4.6. Method Validation
4.7. Pharmacokinetic and Drug–Drug Interaction Study
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Björkström, K.; Franzén, S.; Eliasson, B.; Miftaraj, M.; Gudbjörnsdottir, S.; Trolle-Lagerros, Y.; Svensson, A.; Hagström, H. Risk Factors for Severe Liver Disease in Patients with Type 2 Diabetes. Clin. Gastroenterol. Hepatol. 2019, 17, 2769–2775. [Google Scholar] [CrossRef]
- El-serag, H.B.; Tran, T.; Everhart, J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004, 126, 460–468. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef]
- Liu, L.I.; Cao, Y.; Chen, C.; Zhang, X.; Mcnabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Huang, A.; Yang, X.; Chung, W.; Dennison, A.R.; Zhou, J. Targeted therapy for hepatocellular carcinoma. Signal Transduct. Target. Ther. 2020, 5, 146. [Google Scholar] [CrossRef]
- Keating, G.M.; Santoro, A. Sorafenib: A Review of its Use in Advanced Hepatocellular Carcinoma. Drugs 2009, 69, 223–240. [Google Scholar] [CrossRef]
- Gong, L.; Giacomini, M.M.; Giacomini, C.; Maitland, M.L.; Altman, R.B.; Klein, T.E. PharmGKB summary. Pharmacogenet. Genome 2017, 27, 240–246. [Google Scholar] [CrossRef]
- Edginton, A.N.; Zimmerman, E.I.; Vasilyeva, A.; Baker, S.D.; Panetta, J.C. Sorafenib metabolism, transport, and enterohepatic recycling: Physiologically based modeling and simulation in mice. Cancer Chemother. Pharmacol. 2016, 77, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Mross, K.; Steinbild, S.; Baas, F.; Gmehling, D.; Radtke, M.; Voliotis, D.; Brendel, E.; Christensen, O.; Unger, C. Results from an in vitro and a clinical/pharmacological phase I study with the combination irinotecan and sorafenib. Eur. J. Cancer 2007, 43, 55–63. [Google Scholar] [CrossRef]
- Zimmerman, E.I.; Hu, S.; Roberts, J.L.; Gibson, A.A.; Orwick, S.J.; Li, L.; Sparreboom, A.; Baker, S.D. Contribution of OATP1B1 and OATP1B3 to the Disposition of Sorafenib and Sorafenib-Glucuronide. Clin. Cancer Res. 2013, 19, 1458–1466. [Google Scholar] [CrossRef]
- Vasilyeva, A.; Durmus, S.; Li, L.; Wagenaar, E.; Hu, S.; Gibson, A.A.; Panetta, J.C.; Mani, S.; Sparreboom, A.; Baker, S.D.; et al. Hepatocellular Shuttling and Recirculation of Sorafenib-Glucuronide Is Dependent on Abcc2, Abcc3, and Oatp1a/1b. Cancer Res. 2015, 75, 2729–2736. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Catalano, M.; Casadei-Gardini, A.; Vannini, G.; Campani, C.; Marra, F.; Mini, E.; Roviello, G. Lenvatinib: Established and promising drug for the treatment of advanced hepatocellular carcinoma. Expert Rev. Clin. Pharmacol. 2021, 14, 1353–1365. [Google Scholar] [CrossRef]
- Shumaker, R.; Aluri, J.; Fan, J.; Martinez, G.; Ren, M.; Chen, K. Evaluation of the effects of formulation and food on the pharmacokinetics of lenvatinib (E7080) in healthy volunteers. Int. J. Clin. Pharmacol. Ther. 2014, 52, 284–291. [Google Scholar] [CrossRef]
- Shumaker, R.; Aluri, J.; Fan, J.; Martinez, G.; Thompson, G.A.; Ren, M. Effects of ketoconazole on the pharmacokinetics of lenvatinib (E7080) in healthy participants. Clin. Pharm. Drug Dev. 2015, 4, 155–160. [Google Scholar] [CrossRef]
- Vavrová, K.; Indra, R.; Pompach, P.; Heger, Z.; Hodek, P. The impact of individual human cytochrome P450 enzymes on oxidative metabolism of anticancer drug lenvatinib. Biomed. Pharmacother. 2022, 145, 112391. [Google Scholar] [CrossRef]
- Gupta, A.; Jarzab, B.; Capdevila, J.; Shumaker, R.; Hussein, Z. Population pharmacokinetic analysis of lenvatinib in healthy subjects and patients with cancer. Br. J. Clin. Pharmacol. 2016, 81, 1124–1133. [Google Scholar] [CrossRef] [Green Version]
- Tamai, T.; Hayato, S.; Hojo, S.; Suzuki, T.; Okusaka, T.; Ikeda, K.; Kumada, H. Dose Finding of Lenvatinib in Subjects with Advanced Hepatocellular Carcinoma Based on Population Pharmacokinetic and Exposure-Response Analyses. J. Clin. Pharmacol. 2017, 57, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, R.C.; Aluri, J.; Fan, J.; Martinez, G.; Thompson, G.A.; Ren, M. Effect of rifampicin on the pharmacokinetics of lenvatinib in healthy adults. Clin. Drug Investig. 2014, 34, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Ning, C.; Wang, Z.; Wang, Y.; Zheng, M.; Zhang, S.; Lu, Y.; Zhang, Y.; Li, N.; et al. Influences of ABC transporter and CYP3A4/5 genetic polymorphisms on the pharmacokinetics of lenvatinib in Chinese healthy subjects. Eur. J. Clin. Pharmacol. 2020, 76, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Sodium-glucose cotransporter type 2 inhibitors for the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 556–577. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, R.N.V.S.; Cuyckens, F.; Chen, J.; Scheers, E.; Kalamaridis, D.; Lin, R.; Silva, J.; Sha, S.; Evans, D.C.; Kelley, M.F.; et al. Metabolism and Excretion of Canagliflozin in Mice, Rats, Dogs, and Humans. Drug Metab. Dispos. 2014, 42, 903–916. [Google Scholar] [CrossRef]
- Devineni, D.; Vaccaro, N.; Murphy, J.; Curtin, C.; Mamidi, R.N.V.S.; Weiner, S.; Wang, S.; Ariyawansa, J.; Stieltjes, H.; Wajs, E.; et al. Effects of rifampin, cyclosporine A, and probenecid on the pharmacokinetic profile of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in healthy participants. Int. J. Clin. Pharmacol. Ther. 2015, 53, 115–128. [Google Scholar] [CrossRef]
- Devineni, D.; Polidori, D. Clinical Pharmacokinetic, Pharmacodynamic, and Drug–Drug Interaction Profile of Canagliflozin, a Sodium-Glucose Co-transporter 2 Inhibitor. Clin. Pharmacokinet. 2015, 54, 1027–1041. [Google Scholar] [CrossRef]
- Mamidi, R.N.V.S.; Dallas, S.; Sensenhauser, C.; Lim, H.K.; Scheers, E.; Verboven, P.; Cuyckens, F.; Leclercq, L.; Evans, D.C.; Kelley, M.F.; et al. In vitro and physiologically-based pharmacokinetic based assessment of drug–drug interaction potential of canagliflozin. Br. J. Clin. Pharm. 2017, 83, 1082–1096. [Google Scholar] [CrossRef]
- Lau, K.T.K.; Ng, L.; Wong, J.W.H.; Loong, H.H.F.; Chan, W.W.L.; Lee, C.H.; Wong, C.K.H. Repurposing sodium-glucose co-transporter 2 inhibitors (SGLT2i) for cancer treatment—A Review. Rev. Endocr. Metab. Disord. 2021, 22, 1121–1136. [Google Scholar] [CrossRef]
- Madunić, I.V.; Madunić, J.; Breljak, D.; Karaica, D.; Sabolić, I. Sodium-glucose cotransporters: New targets of cancer therapy? Arh. Hig. Rada Toksikol. 2018, 69, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Kaji, K.; Nishimura, N.; Seki, K.; Sato, S.; Saikawa, S.; Nakanishi, K.; Furukawa, M.; Kawaratani, H.; Kitade, M.; Moriya, K.; et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int. J. Cancer 2018, 142, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Shulman, G.I. Sodium-glucose cotransporter-2 inhibitors: Understanding the mechanisms for therapeutic promise and persisting risks. J. Biol. Chem. 2020, 295, 14379–14390. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.; Chen, Y.; Chen, L.; Chu, P.; Hsieh, F.; Tsai, M.; Shih, C.; Chao, T.; Huang, C.; Chen, K. Canagliflozin inhibits growth of hepatocellular carcinoma via blocking glucose-influx-induced β-catenin activation. Cell Death Dis. 2019, 10, 420. [Google Scholar] [CrossRef]
- Ali, J.; Ali, A. Development and validation of UPLC/ESI-Q-TOF-MS for carteolol in aqueous humour: Stability, stress degradation and application in pharmacokinetics of nanoformulation. Arab. J. Chem. 2017, 10, S2969–S2978. [Google Scholar]
- Karbownik, A.; Szkutnik-Fiedler, D.; Czyrski, A.; Kostewicz, N.; Kaczmarska, P.; Bekier, M.; Stanisławiak-Rudowicz, J.; Karaźniewicz-Łada, M.; Wolc, A.; Główka, F.; et al. Pharmacokinetic Interaction between Sorafenib and Atorvastatin, and Sorafenib and Metformin in Rats. Pharmaceutics 2020, 12, 600. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Liu, F.; Wang, M.; Qin, S. The effects of triptolide on the pharmacokinetics of sorafenib in rats and its potential mechanism. Pharm. Biol. 2017, 55, 1863–1867. [Google Scholar] [CrossRef]
- Reagan Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef]
- Mai, H.; Huang, J.; Zhang, Y.; Qu, N.; Qu, H.; Mei, G.H.; Liu, J.; Xu, X.; Chen, L. In-vivo relation between plasma concentration of sorafenib and its safety in Chinese patients with metastatic renal cell carcinoma: A single-center clinical study. Oncotarget 2017, 8, 43458–43469. [Google Scholar] [CrossRef]
- Fukudo, M.; Ito, T.; Mizuno, T.; Shinsako, K.; Hatano, E.; Uemoto, S.; Kamba, T.; Yamasaki, T.; Ogawa, O.; Seno, H.; et al. Exposure–Toxicity Relationship of Sorafenib in Japanese Patients with Renal Cell Carcinoma and Hepatocellular Carcinoma. Clin. Pharmacokinet. 2014, 53, 185–196. [Google Scholar] [CrossRef]
- Nagahama, M.; Ozeki, T.; Suzuki, A.; Sugino, K.; Niioka, T.; Ito, K.; Miura, M. Association of lenvatinib trough plasma concentrations with lenvatinib-induced toxicities in Japanese patients with thyroid cancer. Med. Oncol. 2019, 36, 39. [Google Scholar] [CrossRef]
- Hata, K.; Suetsugu, K.; Egashira, N.; Makihara, Y.; Itoh, S.; Yoshizumi, T.; Tanaka, M.; Kohjima, M.; Watanabe, H.; Masuda, S.; et al. Association of lenvatinib plasma concentration with clinical efficacy and adverse events in patients with hepatocellular carcinoma. Cancer Chemother. Pharmacol. 2020, 86, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Devineni, D.; Ghosh, A.; Polidori, D.; Chien, S.; Wexler, D.; Shalayda, K.; Demarest, K.; Rothenberg, P. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes. Metab. 2011, 13, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Huang, X.; Li, Y.; Wu, M.; Liu, J. The drug-drug interaction of sorafenib mediated by P-glycoprotein and CYP3A4. Xenobiotica 2016, 46, 651–658. [Google Scholar] [CrossRef]
- Wei, J.; Liu, R.; Zhang, J.; Liu, S.; Yan, D.; Wen, X.; Tian, X. Baicalin Enhanced Oral Bioavailability of Sorafenib in Rats by Inducing Intestine Absorption. Front. Pharmacol. 2021, 12, 761763. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; D’Cunha, R.; Shao, J.; An, G. Effect of 5,7-dimethoxyflavone on Bcrp1-mediated transport of sorafenib in vitro and in vivo in mice. Eur. J. Pharm. Sci. 2018, 117, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Karbownik, A.; Miedziaszczyk, M.; Grabowski, T.; Stanisławiak-Rudowicz, J.; Jaźwiec, R.; Wolc, A.; Grześkowiak, E.; Szałek, E. In vivo assessment of potential for UGT-inhibition-based drug-drug interaction between sorafenib and tapentadol. Biomed. Pharmacother. 2020, 130, 110530. [Google Scholar] [CrossRef]
- Estudante, M.; Morais, J.G.; Soveral, G.; Benet, L.Z. Intestinal drug transporters: An overview. Adv. Drug Deliver. Rev. 2013, 65, 1340–1356. [Google Scholar] [CrossRef]
- Lund, M.; Petersen, T.S.; Dalhoff, K.P. Clinical Implications of P-Glycoprotein Modulation in Drug–Drug Interactions. Drugs 2017, 77, 859–883. [Google Scholar] [CrossRef]
- Chen, L.; Manautou, J.E.; Rasmussen, T.P.; Zhong, X. Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharm. Sin. B 2019, 9, 659–674. [Google Scholar] [CrossRef]
- König, J.; Müller, F.; Fromm, M.F. Transporters and Drug-Drug Interactions: Important Determinants of Drug Disposition and Effects. Pharmacol. Rev. 2013, 65, 944–966. [Google Scholar] [CrossRef] [Green Version]
- Fredrick, C.A.; Ernest, C.A.; Hitler, L.; Maryjane, C.M.; Innocent, B.; Terkumbur, E.G.; Gideon, E.M.; Adedapo, S.A.; Alexander, I.I. Structural benchmarking, density functional theory simulation, spectroscopic investigation and molecular docking of N-(1H-pyrrol-2-yl) methylene)-4-methylaniline as castration-resistant prostate cancer chemotherapeutic agent. Chem. Phys. Impact. 2022, 5, 100091. [Google Scholar]
- Izuagbe, G.O.; Emmanuella, E.O.; Hitler, L.; Khan, E.M.; Emmanuel, E.E.; Henry, O.E.; Onyinye, J.I.; Amoawe, P.O.; Faith, O. Antibacterial potential of N-(2-furylmethylidene)-1, 3, 4-thiadiazole-2-amine: Experimental and theoretical investigations. J. Indian Chem. Soc. 2022, 99, 100597. [Google Scholar]
- Ugwu, D.I.; Fredrick, C.A.; Hitler, L.; Eze, F.U.; Terkumbur, E.G.; Ugwu, M.C.; Ndefo, J.C.; Benedeth, O.E.; Adedapo, S.A.; Okoro, U.C. Synthesis, vibrational analysis, molecular property investigation, and molecular docking of new benzenesulphonamide-based carboxamide derivatives against Plasmodium falciparum. J. Mol. Struct. 2022, 1269, 133796. [Google Scholar]

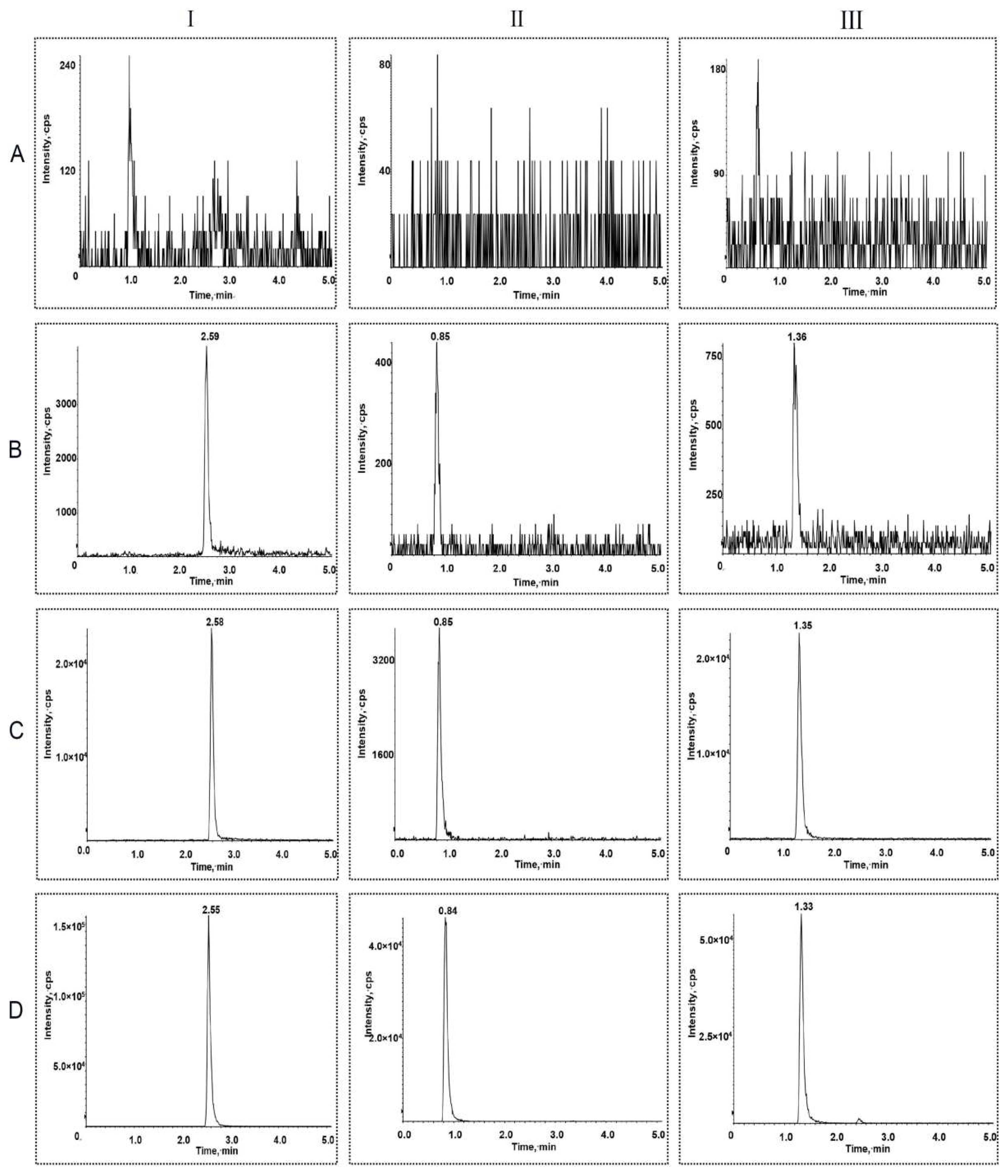

| Analytes | Concentration (ng/mL) | Intra-Batch (n = 6) | Inter-Batch (n = 18) | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | CV (%) | RE (%) | Mean ± SD | CV (%) | RE (%) | ||
| Sorafenib | 5 | 5.32 ± 0.29 | 5.42 | 6.30 | 5.48 ± 0.26 | 4.72 | 9.57 |
| 10 | 10.08 ± 0.59 | 5.86 | 0.80 | 10.60 ± 0.60 | 5.70 | 6.04 | |
| 1500 | 1481.67 ± 84.72 | 5.72 | −1.22 | 1471.67 ± 97.03 | 6.59 | −1.89 | |
| 3750 | 3900.00 ± 264.20 | 6.77 | 4.00 | 3855.59 ± 258.05 | 6.69 | 2.81 | |
| Lenvatinib | 0.2 | 0.21 ± 0.02 | 9.54 | 6.08 | 0.22 ± 0.02 | 8.24 | 8.03 |
| 0.5 | 0.52 ± 0.02 | 4.76 | 3.20 | 0.51 ± 0.04 | 7.51 | 2.43 | |
| 150 | 147.67 ± 5.92 | 4.01 | −1.56 | 149.33 ± 10.13 | 6.78 | −0.44 | |
| 750 | 715.83 ± 30.51 | 4.26 | −4.56 | 738.78 ± 43.23 | 5.85 | −1.50 | |
| Canagliflozin | 5 | 5.49 ± 0.21 | 3.75 | 9.73 | 5.26 ± 0.42 | 7.93 | 5.10 |
| 10 | 10.36 ± 0.46 | 4.43 | 3.55 | 10.31 ± 0.59 | 5.70 | 3.06 | |
| 800 | 782.83 ± 42.24 | 5.40 | −2.15 | 787.28 ± 66.44 | 8.44 | −1.59 | |
| 2250 | 2155.00 ± 103.10 | 4.78 | −4.22 | 2200.56 ± 119.63 | 5.44 | −2.20 | |
| Analytes | Concentration (ng/mL) | Matrix Effect | Extraction Recovery | ||
|---|---|---|---|---|---|
| Mean ± SD (%) | CV (%) | Mean ± SD (%) | CV (%) | ||
| Sorafenib | 10 | 105.44 ±4.81 | 4.57 | 97.55 ± 4.03 | 4.13 |
| 1500 | 99.57 ± 7.01 | 7.04 | 100.18 ± 3.67 | 3.66 | |
| 3750 | 99.98 ± 5.08 | 5.08 | 100.59 ± 6.84 | 6.80 | |
| Lenvatinib | 0.5 | 103.74 ± 11.20 | 10.80 | 89.97 ± 8.48 | 9.43 |
| 150 | 95.67 ± 10.44 | 10.91 | 100.08 ± 9.47 | 9.47 | |
| 800 | 96.65 ± 2.17 | 2.25 | 99.40 ± 9.27 | 9.33 | |
| Canagliflozin | 10 | 101.05 ± 9.13 | 9.03 | 97.07 ± 9.49 | 9.78 |
| 800 | 95.82 ± 7.21 | 7.52 | 100.25 ± 7.81 | 7.79 | |
| 2250 | 103.16 ± 3.72 | 3.61 | 96.85 ± 6.22 | 6.42 | |
| Analytes | Concentration (ng/mL) | Bench-Top a | Autosampler b | Freeze-Thaw c | Long-Term d |
|---|---|---|---|---|---|
| Sorafenib | 10 | 10.97 ± 0.29 | 11.13 ± 0.24 | 10.55 ± 0.43 | 10.51 ± 0.43 |
| 1500 | 1551.67 ± 84.00 | 1540.00 ± 95.71 | 1531.67 ± 117.88 | 1565.00 ± 123.25 | |
| 3750 | 3948.33 ± 99.88 | 3961.67 ± 102.26 | 3698.33 ± 72.78 | 3583.33 ± 67.43 | |
| Lenvatinib | 0.5 | 0.54 ± 0.03 | 0.53 ± 0.04 | 0.53 ± 0.03 | 0.47 ± 0.03 |
| 150 | 156.67 ± 7.42 | 162.67 ± 7.42 | 157.33 ± 11.02 | 157.00 ± 12.63 | |
| 800 | 794.00 ± 18.24 | 819.50 ± 30.36 | 725.83 ± 18.06 | 714.00 ± 24.44 | |
| Canagliflozin | 10 | 10.87 ± 0.52 | 11.13 ± 0.23 | 9.60 ± 0.45 | 10.02 ± 0.71 |
| 800 | 849.83 ± 44.57 | 847.67 ± 52.93 | 854.50 ± 48.73 | 830.00 ± 42.37 | |
| 2250 | 2330.00 ± 80.00 | 2350.00 ± 72.94 | 2183.33 ± 82.62 | 2170.00 ± 59.67 |
| Parameters (Unit) | ISOR | IVSOR+CA | p-Value |
|---|---|---|---|
| t1/2z (h) | 12.30 ± 2.23 | 10.47 ± 0.94 | 0.109 |
| Cmax (ng/mL) | 1916 ± 298 | 2195 ± 535 | 0.292 |
| AUClast (h·ng/mL) | 39,970 ± 14,538 | 41,158 ± 9197 | 0.869 |
| AUCinf (h·ng/mL) | 40,273 ± 14,696 | 41,290 ± 9225 | 0.889 |
| Tmax (h) | 8.00 (7.00–8.00) | 6.50 (5.50–7.25) | 0.065 |
| CLz/F (L/h/kg) | 2.86 ± 1.30 | 2.52 ± 0.53 | 0.569 |
| Vz/F (L/kg) | 49.00 ± 18.83 | 38.30 ± 10.12 | 0.248 |
| Parameters (Unit) | IIICA | IVSOR+CA | VLEN+CA | IIICA vs. IVSOR+CA p-Value | IIICA vs. VLEN+CA p-Value |
|---|---|---|---|---|---|
| t1/2z (h) | 8.84 ± 0.35 | 8.56 ± 0.61 | 10.12 ± 3.59 | 0.347 | 0.394 |
| Cmax (ng/mL) | 3096 ± 776 | 4103 ± 335 | 3008 ± 484 | 0.041 * | 0.818 |
| AUClast (h·ng/mL) | 48,875 ± 7446 | 67,320 ± 3637 | 67,970 ± 18,572 | 0.002 ** | 0.054 |
| AUCinf (h·ng/mL) | 49,103 ± 7508 | 67,554 ± 3597 | 68,654 ± 19,418 | 0.002 ** | 0.058 |
| Tmax (h) | 3.50 (3.00–6.25) | 5.50 (3.50–6.00) | 4.00 (3.74–4.50) | 0.699 | 0.818 |
| CLz/F (L/h/kg) | 0.21 ± 0.03 | 0.15 ± 0.01 | 0.15 ± 0.04 | 0.002 ** | 0.029 * |
| Vz/F (L/kg) | 2.65 ± 0.39 | 1.84 ± 0.22 | 2.12 ± 0.32 | 0.001 ** | 0.030 * |
| Parameters (Unit) | IILEN | VLEN+CA | p-Value |
|---|---|---|---|
| t1/2z (h) | 10.48 ± 4.53 | 6.49 ± 1.04 | 0.004 ** |
| Cmax (ng/mL) | 721 ± 144 | 985 ± 162 | 0.014 * |
| AUClast (h·ng/mL) | 5640 ± 1292 | 7281 ± 1167 | 0.044 * |
| AUCinf (h·ng/mL) | 5653 ± 1299 | 7285 ± 1167 | 0.045 * |
| Tmax (h) | 1.75 (1.38–2.75) | 1.50 (0.88–1.63) | 0.240 |
| CLz/F (L/h/kg) | 0.22 ± 0.05 | 0.17 ± 0.03 | 0.047 * |
| Vz/F (L/kg) | 3.31 ± 1.51 | 1.56 ± 0.29 | 0.004 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Li, Y.; Guo, C.; Li, Y.; Ma, Y.; Dong, Z. Pharmacokinetic Interactions between Canagliflozin and Sorafenib or Lenvatinib in Rats. Molecules 2022, 27, 5419. https://doi.org/10.3390/molecules27175419
Cui Y, Li Y, Guo C, Li Y, Ma Y, Dong Z. Pharmacokinetic Interactions between Canagliflozin and Sorafenib or Lenvatinib in Rats. Molecules. 2022; 27(17):5419. https://doi.org/10.3390/molecules27175419
Chicago/Turabian StyleCui, Yanjun, Ying Li, Caihui Guo, Yajing Li, Yinling Ma, and Zhanjun Dong. 2022. "Pharmacokinetic Interactions between Canagliflozin and Sorafenib or Lenvatinib in Rats" Molecules 27, no. 17: 5419. https://doi.org/10.3390/molecules27175419






