Development of UPLC-MS/MS Method to Study the Pharmacokinetic Interaction between Sorafenib and Dapagliflozin in Rats
Abstract
:1. Introduction
2. Results
2.1. Method Validation
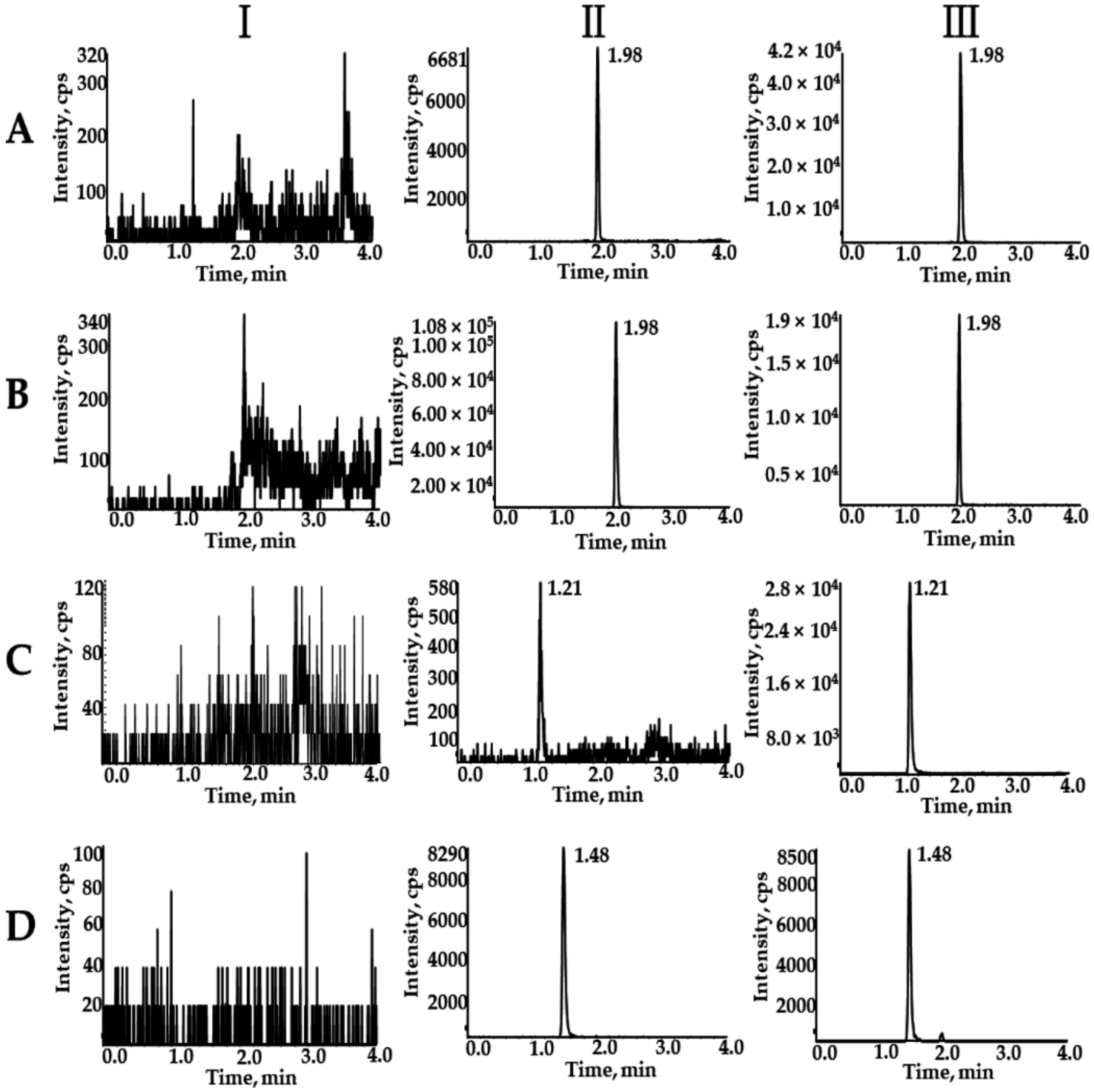
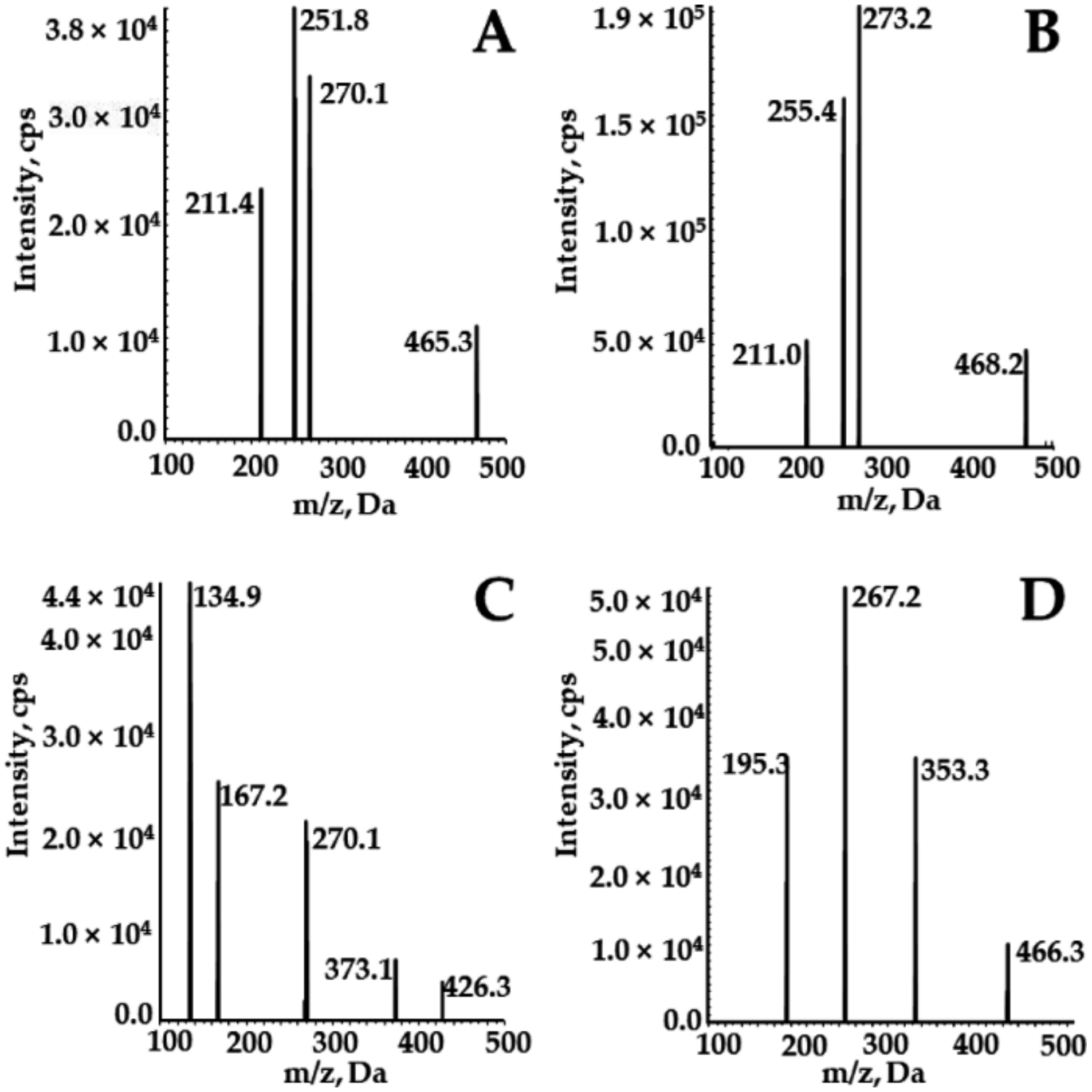
2.1.1. Selectivity
2.1.2. Calibration Curve and LLOQ
2.1.3. Precision and Accuracy
2.1.4. Matrix Effects and Extraction Recovery
2.1.5. Stability
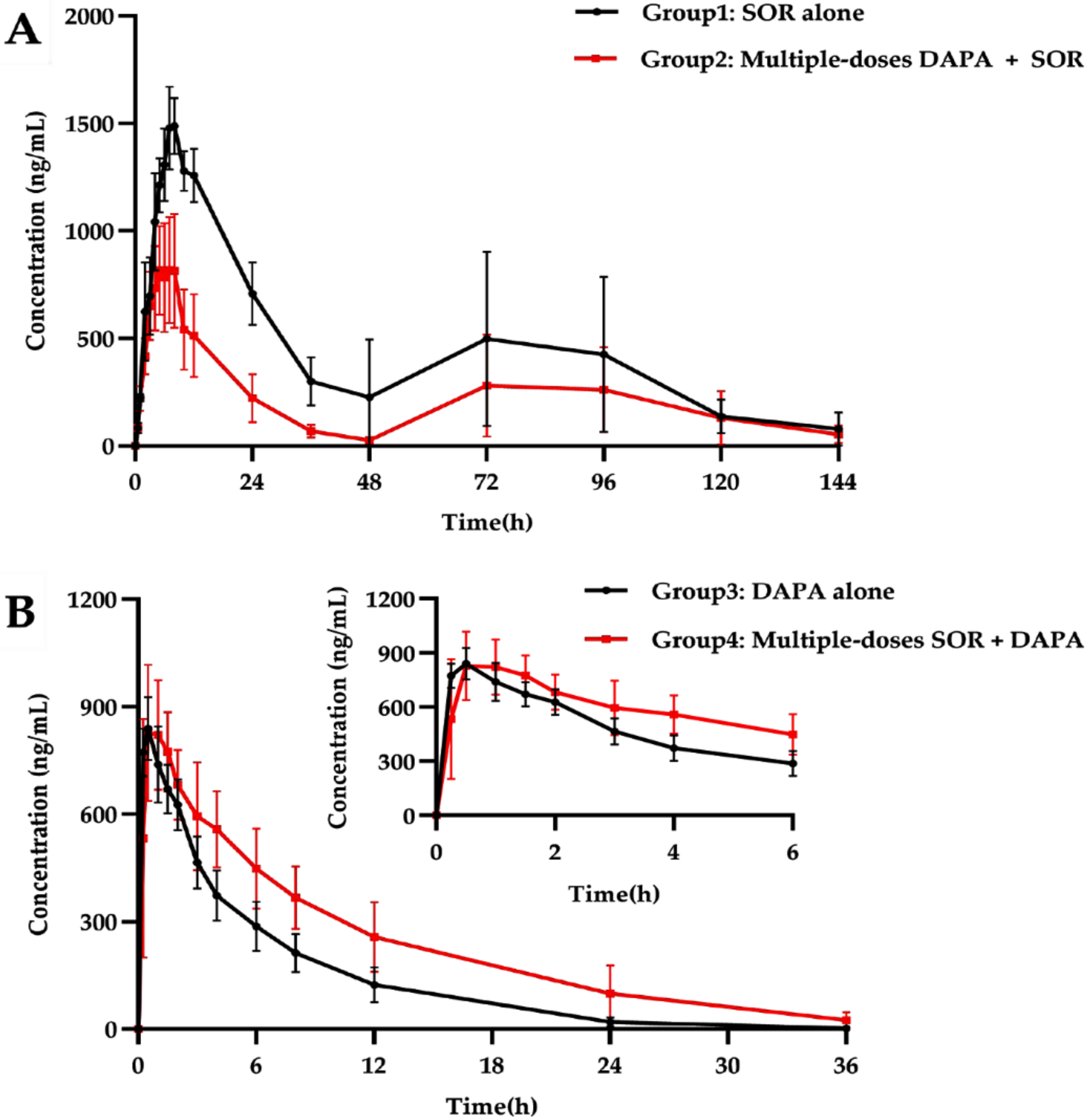
2.2. Pharmacokinetic Interactions between SOR and DAPA
2.2.1. Effect of DAPA on SOR Pharmacokinetics
2.2.2. Effect of SOR on DAPA Pharmacokinetics
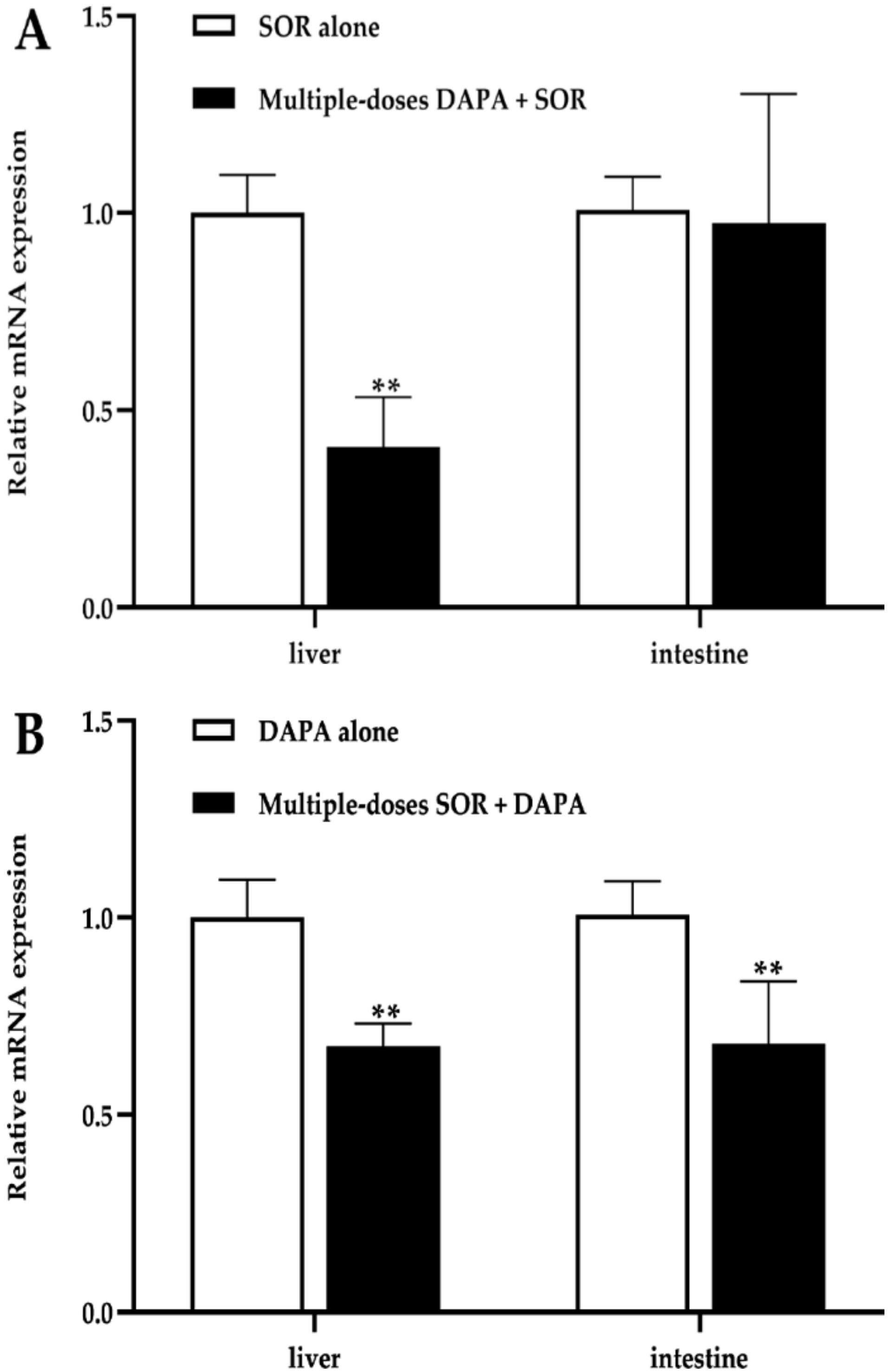
2.3. mRNA Expression in the Liver and Intestines
3. Discussion
4. Materials and Methods
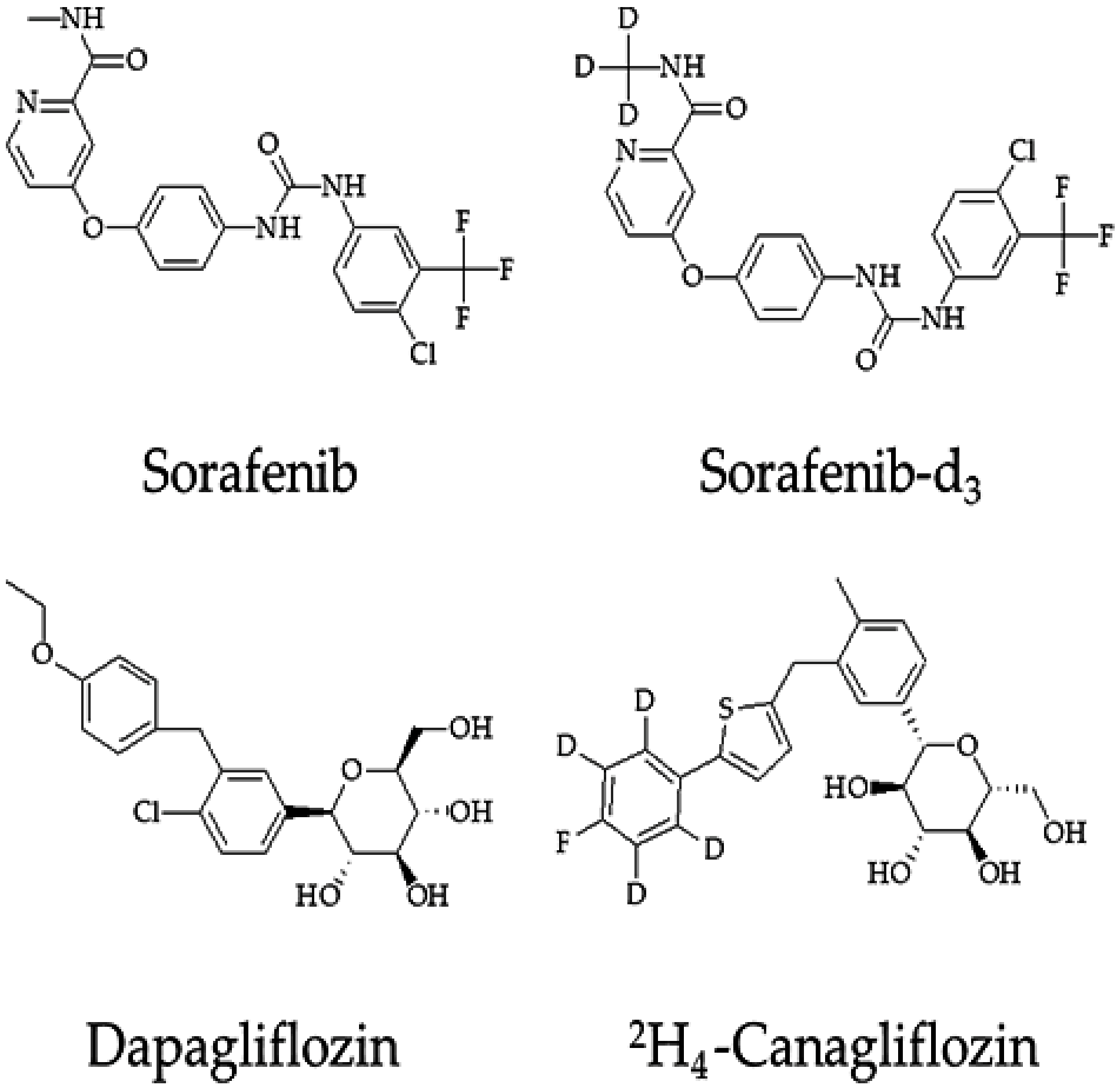
4.1. Chemicals and Regents
4.2. Instrumentation and Chromatographic Conditions
4.3. Preparation of Stock Solution and Working Solution
4.4. Preparation of Calibration Standards and Quality Control (QC) Samples
4.5. Plasma Sample Preparation
4.6. Method Validation
4.6.1. Selectivity
4.6.2. Calibration Curve and LLOQ
4.6.3. Precision and Accuracy
4.6.4. Matrix Effects and Extraction Recovery
4.6.5. Stability
4.7. Pharmacokinetic Experiments in Rats
4.8. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mcglynn, K.A.; Petrick, J.L.; El Serag, H.B. Epidemiology of hepatocellular carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Das, B.K.; Choudhary, S.; Gupta, D.; Patil, U.K. Diabetes and hepatocellular carcinoma: A pathophysiological link and pharmacological management. Biomed. Pharmacother. 2018, 106, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wei, S.; Zhang, W.; Yang, J.; Yang, J.; Yan, L. Type 2 diabetes mellitus increases the risk of hepatocellular carcinoma in subjects with chronic hepatitis B virus infection: A meta-analysis and systematic review. Cancer Manag. Res. 2019, 11, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Raoul, J.; Kudo, M.; Finn, R.S.; Edeline, J.; Reig, M.; Galle, P.R. Systemic therapy for intermediate and advanced hepatocellular carcinoma: Sorafenib and beyond. Cancer Treat. Rev. 2018, 68, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef]
- Rowland, A.; van Dyk, M.; Mangoni, A.A.; Miners, J.O.; Mckinnon, R.A.; Wiese, M.D.; Rowland, A.; Kichenadasse, G.; Gurney, H.; Sorich, M.J. Kinase inhibitor pharmacokinetics: Comprehensive summary and roadmap for addressing inter-individual variability in exposure. Expert Opin. Drug Metab. 2017, 13, 31–49. [Google Scholar] [CrossRef]
- Josephs, D.H.; Fisher, D.S.; Spicer, J.; Flanagan, R.J. Clinical pharmacokinetics of tyrosine kinase inhibitors: Implications for therapeutic drug monitoring. Ther. Drug Monit. 2013, 35, 562–587. [Google Scholar] [CrossRef]
- Thomas-Schoemann, A.; Blanchet, B.; Bardin, C.; Noé, C.; Boudou-Rouquette, P.; Vidal, M.; Goldwasser, F. Drug interactions with solid tumour-targeted therapies. Crit. Rev. Oncol. Hematol. 2014, 89, 179–196. [Google Scholar] [CrossRef]
- Swift, B.; Nebot, N.; Lee, J.K.; Han, T.; Proctor, W.R.; Thakker, D.R.; Lang, D.; Radtke, M.; Gnoth, M.J.; Brouwer, K.L.R. Sorafenib hepatobiliary disposition: Mechanisms of hepatic uptake and disposition of generated metabolites. Drug Metab. Dispos. 2013, 41, 1179–1186. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Huang, X.; Li, Y.; Wu, M.; Liu, J. The drug-drug interaction of sorafenib mediated by P-glycoprotein and CYP3A4. Xenobiotica 2016, 46, 651–658. [Google Scholar] [CrossRef]
- Karbownik, A.; Miedziaszczyk, M.; Grabowski, T.; Stanisławiak-Rudowicz, J.; Jaźwiec, R.; Wolc, A.; Grześkowiak, E.; Szałek, E. In vivo assessment of potential for UGT-inhibition-based drug-drug interaction between sorafenib and tapentadol. Biomed. Pharmacother. 2020, 130, 110530. [Google Scholar] [CrossRef]
- Karbownik, A.; Szkutnik-Fiedler, D.; Czyrski, A.; Kostewicz, N.; Kaczmarska, P.; Bekier, M.; Stanisławiak-Rudowicz, J.; Karaźniewicz-Łada, M.; Wolc, A.; Główka, F.; et al. Pharmacokinetic interaction between sorafenib and atorvastatin, and sorafenib and metformin in rats. Pharmaceutics 2020, 12, 600. [Google Scholar] [CrossRef]
- Miners, J.O.; Chau, N.; Rowland, A.; Burns, K.; Mckinnon, R.A.; Mackenzie, P.I.; Tucker, G.T.; Knights, K.M.; Kichenadasse, G. Inhibition of human UDP-glucuronosyltransferase enzymes by lapatinib, pazopanib, regorafenib and sorafenib: Implications for hyperbilirub. Biochem. Pharmacol. 2017, 129, 85–95. [Google Scholar] [CrossRef]
- Mross, K.; Steinbild, S.; Baas, F.; Gmehling, D.; Radtke, M.; Voliotis, D.; Brendel, E.; Christensen, O.; Unger, C. Results from an in vitro and a clinical/pharmacological phaseⅠstudy with the combination irinotecan and sorafenib. Eur. J. Cancer 2007, 43, 55–63. [Google Scholar] [CrossRef]
- Peer, C.J.; Sissung, T.M.; Kim, A.; Jain, L.; Woo, S.; Gardner, E.R.; Kirkland, C.T.; Troutman, S.M.; English, B.C.; Richardson, E.D.; et al. Sorafenib is an inhibitor of UGT1A1 but is metabolized by UGT1A9: Implications of genetic variants on pharmacokinetics and hyperbilirubinemia. Clin. Cancer Res. 2012, 18, 2099–2107. [Google Scholar] [CrossRef]
- Dhillon, S. Dapagliflozin: A review in type 2 diabetes. Drugs 2019, 79, 1135–1146. [Google Scholar] [CrossRef]
- Kluger, A.Y.; Tecson, K.M.; Lee, A.Y.; Lerma, E.V.; Rangaswami, J.; Lepor, N.E.; Cobble, M.E.; Mccullough, P.A. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc. Diabetol. 2019, 18, 99. [Google Scholar] [CrossRef]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Murohisa, T.; Lijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; Aso, Y. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 285–292. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Csermely, A.; Beatrice, G.; Targher, G. Sodium-glucose cotransporter-2 inhibitors for treatment of nonalcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Metabolites 2021, 11, 22. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Huang, W.; Han, Y.; Tan, H.; An, M.; Xiang, Q.; Zhou, R.; Yang, L.; Cheng, Y. Dapagliflozin alleviates hepatic steatosis by restoring autophagy via the AMPK-mTOR pathway. Front. Pharmacol. 2021, 12, 589273. [Google Scholar] [CrossRef]
- Kasichayanula, S.; Liu, X.; Lacreta, F.; Griffen, S.C.; Boulton, D.W. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin. Pharmacokinet. 2014, 53, 17–27. [Google Scholar] [CrossRef]
- Kasichayanula, S.; Liu, X.; Griffen, S.G.; Lacreta, F.P.; Boulton, D.W. Effects of rifampin and mefenamic acid on the pharmacokinetics and pharmacodynamics of dapagliflozin. Diabetes Obes. Metab. 2013, 15, 280–283. [Google Scholar] [CrossRef]
- Pattanawongsa, A.; Chau, N.; Rowland, A.; Miners, J.O. Inhibition of human UDP-glucuronosyltransferase enzymes by canagliflozin and dapagliflozin: Implications for drug-drug interactions. Drug Metab. Dispos. 2015, 43, 1468–1476. [Google Scholar] [CrossRef]
- Jain, L.; Gardner, E.R.; Venitz, J.; Dahut, W.; Figg, W.D. Development of a rapid and sensitive LC–MS/MS assay for the determination of sorafenib in human plasma. J. Pharm. Biomed. Anal. 2008, 46, 362–367. [Google Scholar] [CrossRef]
- He, Y.; Zhou, L.; Gao, S.; Yin, T.; Tu, Y.; Rayford, R.; Wang, X.; Hu, M. Development and validation of a sensitive LC-MS/MS method for simultaneous determination of eight tyrosine kinase inhibitors and its application in mice pharmacokinetic studies. J. Pharm. Biomed. Anal. 2018, 148, 65–72. [Google Scholar] [CrossRef]
- Heinz, W.J.; Kahle, K.; Helle-Beyersdorf, A.; Schirmer, D.; Lenker, U.; Keller, D.; Langmann, P.; Klinker, H. High-performance liquid chromatographic method for the determination of sorafenib in human serum and peritoneal fluid. Cancer Chemother. Pharmacol. 2011, 68, 239–245. [Google Scholar] [CrossRef]
- Iacuzzi, V.; Zanchetta, M.; Gagno, S.; Poetto, A.S.; Orleni, M.; Marangon, E.; Guardascione, M.; Foltran, L.; Posocco, B.; Toffoli, G. A LC–MS/MS method for therapeutic drug monitoring of sorafenib, regorafenib and their active metabolites in patients with hepatocellular carcinoma. J. Pharm. Biomed. Anal. 2020, 187, 113358. [Google Scholar] [CrossRef]
- Van der Aart-van der Beek, A.B.; Wessels, A.M.A.; Heerpink, H.J.L.; Touw, D.J. Simple, fast and robust LC-MS/MS method for the simultaneous quantification of canagliflozin, dapagliflozin and empagliflozin in human plasma and urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1152, 122257. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.A.; Shrivastav, P.S.; Shah, J.V.; George, A. Simultaneous quantitation of metformin and dapagliflozin in human plasma by LC–MS/MS: Application to a pharmacokinetic study. Biomed. Chromatogr. 2019, 33, e4453. [Google Scholar] [CrossRef] [PubMed]
- Dias, B.C.L.; Fachi, M.M.; de Campus, M.L.; Degaut, F.L.D.; Peccinini, R.G.; Pontarolo, R. A new HPLC-MS/MS method for the simultaneous quantification of sglt2 inhibitors and metformin in plasma and its application to a pharmacokinetic study in healthy volunteers. Biomed. Chromatogr. 2019, 33, e4663. [Google Scholar] [CrossRef] [PubMed]
- Aubry, A.F.; Gu, H.; Magnier, R.; Morgan, L.; Xu, X.; Timenstein, M.; Wanf, B.; Deng, Y.; Cai, J.; Couerbe, P.; et al. Validated LC-MS/MS methods for the determination of dapagliflozin, a sodium-glucose co-transporter 2 inhibitor in normal and ZDF rat plasma. Bioanalysis 2010, 2, 2001–2009. [Google Scholar] [CrossRef]
- Webb, L.J.; Miles, K.K.; Auyeung, D.J.; Kessler, F.K.; Ritter, J.K. Analysis of substrate specificities and tissue expression of rat UDP-glucuronosyltransferases ugt1a7 and ugt1a8. Drug Metab. Dispos. 2004, 33, 77–82. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, M.; Niu, H.; Jiang, K.; Jiang, J.; Ma, Y.; Wang, X.; Meng, S. Upregulation of UDP-Glucuronosyltransferases 1a1 and 1a7 Aer Involved in Altered Puerarin Pharmacokinetics in Type II Diabetic Rats. Molecules 1018, 23, 1487. [Google Scholar] [CrossRef]
- Koni, A.A.; Nazzal, M.A.; Suwan, B.A.; Sobuh, S.S.; Abuhazeem, N.T.; Salman, A.N.; Salameh, H.T.; Amer, R.; Zyoud, S.E.H. A comprehensive evaluation of potentially significant drug-drug, drug-herb, and drug-food interactions among cancer patients receiving anticancer drugs. BMC Cancer 2022, 22, 547. [Google Scholar] [CrossRef]
- Casadei Gardini, A.; Marisi, G.; Scarpi, E.; Scartozzi, M.; Faloppi, L.; Silvestris, N.; Masi, G.; Vivaldi, C.; Brunetti, O.; Tamberi, S.; et al. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert Opin. Pharmacother. 2015, 16, 2719–2725. [Google Scholar] [CrossRef]
- Rimassa, L.; Danesi, R.; Pressiani, T.; Merle, P. Management of adverse events associated with tyrosine kinase inhibitors: Improving outcomes for patients with hepatocellular carcinoma. Cancer Treat. Rev. 2019, 77, 20–28. [Google Scholar] [CrossRef]
- Tang, W.; Reele, S.; Hamer-Maansson, J.E.; Parikh, S.; de Bruin, T.W.A. Dapagliflozin twice daily or once daily: Effect on pharmacokinetics and urinary glucose excretion in healthy subjects. Diabetes Obes. Metab. 2015, 17, 423–425. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, Z.; Thapa, P.; Jin, Y.S.; Park, J.W.; Lim, H.J.; Lee, J.Y.; Lee, S.W.; Seo, M.H.; Kim, M.S.; et al. Development of sorafenib loaded nanoparticles to improve oral bioavailability using a quality by design approach. Int. J. Pharm. 2019, 566, 229–238. [Google Scholar] [CrossRef]
- Ji, Q.C.; Xu, X.; Ma, E.; Liu, J.; Basdeo, S.; Liu, G.; Mylott, W.; Boulton, D.W.; Shen, J.X.; Stouffer, B.; et al. Selective reaction monitoring of negative electrospray ionization acetate adduct ions for the bioanalysis of dapagliflozin in clinical studies. Anal. Chem. 2015, 87, 3247–3254. [Google Scholar] [CrossRef] [PubMed]
- Reagan Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Bins, S.; van Doorn, L.; Phelps, M.A.; Gibson, A.A.; Hu, S.; Li, L.; Vasilyeva, A.; Du, G.; Hamberg, P.; Eskens, F.; et al. Influence of oatp1b1 function on the disposition of sorafenib-β-d-glucuronide. Clin. Transl. Sci. 2017, 10, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Battson, M.L.; Jarrell, D.K.; Hou, S.; Ecton, K.E.; Weir, T.L.; Gentile, C.L. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc. Diabetol. 2018, 17, 62. [Google Scholar] [CrossRef]
- Yang, M.; Shi, F.; Liu, W.; Zhang, M.; Feng, R.; Qian, C.; Liu, W.; Ma, J. Dapagliflozin modulates the fecal microbiota in a type 2 diabetic rat model. Front. Endocrinol. 2020, 11, 635. [Google Scholar] [CrossRef]
- European Medicines Agency. Summary of Product Characteristic for Nexavar 200 mg. Available online: https://www.ema.europa.eu/en/documents/product-information/nexavar-epar-product-information_en.pdf (accessed on 17 September 2022).
- Scheen, A.J. Drug-drug interactions with sodium-glucose cotransporters type 2 (SGLT2) inhibitors, new oral glucose-lowering agents for the management of type 2 diabetes mellitus. Clin. Pharmacokinet. 2014, 53, 295–304. [Google Scholar] [CrossRef] [Green Version]
| Analytes | Concentration (ng/mL) | Intra-Day (n = 6) | Inter-Day (n = 18) | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | RSD (%) | RE (%) | Mean ± SD | RSD (%) | RE (%) | ||
| SOR | 5 | 5.14 ± 0.27 | 5.3 | 2.7 | 5.03 ± 0.27 | 5.5 | 0.6 |
| 10 | 10.35 ± 0.47 | 4.6 | 3.5 | 10.22 ± 0.51 | 5.0 | 2.2 | |
| 1500 | 1538.33 ± 31.89 | 2.1 | 2.6 | 1558.33 ± 82.69 | 5.3 | 3.9 | |
| 3750 | 3786.67 ± 77.11 | 2.0 | 1.0 | 3745.56 ± 150.30 | 4.0 | −0.1 | |
| DAPA | 5 | 5.16 ± 0.24 | 4.6 | 3.2 | 5.12 ± 0.31 | 6.1 | 2.3 |
| 15 | 15.55 ± 0.80 | 5.1 | 3.7 | 15.42 ± 0.70 | 4.6 | 2.8 | |
| 800 | 824.50 ± 14.40 | 1.8 | 3.1 | 815.33 ± 28.31 | 3.5 | 1.9 | |
| 1500 | 1546.67 ± 52.03 | 3.4 | 3.1 | 1523.89 ± 95.37 | 6.3 | 1.6 | |
| Analytes | Concentration (ng/mL) | Matrix Effect | Extraction Recovery | ||
|---|---|---|---|---|---|
| Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | ||
| SOR | 10 | 100.30 ± 3.30 | 3.3 | 97.29 ± 3.54 | 3.6 |
| 1500 | 96.97 ± 8.80 | 9.1 | 106.94 ± 3.90 | 3.7 | |
| 3750 | 100.78 ± 4.17 | 4.1 | 100.41 ± 2.67 | 2.7 | |
| DAPA | 15 | 106.20 ± 5.61 | 5.3 | 91.80 ± 2.78 | 3.0 |
| 800 | 103.86 ± 4.47 | 4.3 | 104.94 ± 4.16 | 4.0 | |
| 1500 | 101.33 ± 4.65 | 4.6 | 105.44 ± 3.44 | 3.3 | |
| Analytes | Conditions | Concentration (ng/mL) | Mean ± SD (ng/mL) | Precision (RSD%) | Accuracy (RE%) |
|---|---|---|---|---|---|
| SOR | Autosampler for 6 h | 10 | 10.09 ± 0.53 | 5.3 | 0.9 |
| 1500 | 1538.33 ± 36.56 | 2.4 | 2.6 | ||
| 3750 | 3716.67 ± 66.23 | 1.8 | −0.9 | ||
| Room temperature for 4 h | 10 | 10.68 ± 0.55 | 5.2 | 6.8 | |
| 1500 | 1448.33 ± 18.35 | 1.3 | −3.4 | ||
| 3750 | 3533.33 ± 66.23 | 1.9 | −5.8 | ||
| −80 °C for 30 days | 10 | 10.20 ± 0.39 | 3.8 | 2.0 | |
| 1500 | 1533 ± 40.33 | 2.6 | 2.2 | ||
| 3750 | 3593.33 ± 142.22 | 4.0 | −4.2 | ||
| Freeze–thaw stability for three times | 10 | 9.84 ± 0.34 | 3.4 | −1.7 | |
| 1500 | 1550.00 ± 12.65 | 0.8 | 3.3 | ||
| 3750 | 3601.67 ± 164.00 | 4.6 | −4.0 | ||
| DAPA | Autosampler for 6 h | 15 | 14.93 ± 0.83 | 5.5 | −0.4 |
| 800 | 830.00 ± 15.05 | 1.8 | 3.8 | ||
| 1500 | 1643.33 ± 32.66 | 2.0 | 9.6 | ||
| Room temperature for 4 h | 15 | 14.90 ± 0.49 | 3.3 | −0.7 | |
| 800 | 889.00 ± 15.43 | 1.7 | 11.1 | ||
| 1500 | 1653.33 ± 21.60 | 1.3 | 10.2 | ||
| −80 °C for 30 days | 15 | 15.43 ± 1.07 | 6.9 | 2.9 | |
| 800 | 822.17 ± 21.66 | 2.6 | 2.8 | ||
| 1500 | 1483.33 ± 19.66 | 1.3 | −1.1 | ||
| Freeze–thaw stability for three times | 15 | 15.18 ± 0.87 | 5.7 | 1.2 | |
| 800 | 769.83 ± 44.56 | 5.8 | −3.8 | ||
| 1500 | 1388.33 ± 54.19 | 3.9 | −7.4 |
| Parameters (Unit) | SOR (100 mg/kg) | DAPA (1 mg/kg) | ||
|---|---|---|---|---|
| Alone | With Multiple-Doses DAPA | Alone | With Multiple-Doses SOR | |
| AUC0-t (μg/L × h) | 62,701.33 ± 16,697.65 | 31,044.48 ± 11,555.87 ** | 5156.50 ± 1028.25 | 8572.00 ± 2861.57 ** |
| AUC0–∞ (μg/L × h) | 63,708.42 ± 17,561.72 | 34,299.86 ± 12,031.05 ** | 5180.03 ± 1048.61 | 9300.01 ± 4261.40 ** |
| Cmax (μg/L) | 1547.87 ± 136.94 | 904.59 ± 249.56 ** | 846.00 ± 76.39 | 870.83 ± 166.87 |
| Tmax (h) | 7.50 ± 0.55 | 6.00 ± 1.27 * | 0.58 ± 0.20 | 0.83 ± 0.41 ** |
| t1/2z (h) | 17.456 ± 5.65 | 24.82 ± 20.13 | 4.51 ± 0.67 | 7.95 ± 4.03 ** |
| CLz/F (L/h/kg) | 1.69 ± 0.52 | 3.35 ± 1.52 * | 0.20 ± 0.04 | 0.12 ± 0.03 ** |
| Vz/F (L/kg) | 37.43 ± 9.78 | 106.53 ± 69.61 * | 1.27 ± 0.19 | 1.12 ± 0.10 |
| MRT0-t (h) | 47.08 ± 9.48 | 54.99 ± 13.07 | 6.10 ± 0.96 | 9.01 ± 1.22 ** |
| MRT0–∞ (h) | 48.73 ± 10.48 | 67.64 ± 33.94 | 6.25 ± 1.07 | 11.22 ± 4.86 ** |
| Experimental Setting | SOR | DAPA | SOR-d3 | 2H4-DAPA |
|---|---|---|---|---|
| MRM transition | 465.3→270.1 | 426.1→167.2 | 468.2→255.4 | 466.3→195.3 |
| Delustering potential (DP), V | 100 | 80 | 100 | 100 |
| Collision energy (CE), V | 45 | 30 | 45 | 45 |
| Collision cell exit potential (CXP), V | 7 | 7 | 7 | 7 |
| Entrance potential (EP), V | 10 | 10 | 10 | 10 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Ugt1a7 | 5′ AGTGTCCGTTTGGTTGTT-3′ | 5′-TTCCATCGCTTTCTTCTC-3′ |
| NAPDH | 5′-GCCTTCCGTGTTCCTACC-3′ | 5′-GCCTGCTTCACCACCTTC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Li, Y.; Ma, Y.; Fu, Y.; Xun, X.; Cui, Y.; Dong, Z. Development of UPLC-MS/MS Method to Study the Pharmacokinetic Interaction between Sorafenib and Dapagliflozin in Rats. Molecules 2022, 27, 6190. https://doi.org/10.3390/molecules27196190
He X, Li Y, Ma Y, Fu Y, Xun X, Cui Y, Dong Z. Development of UPLC-MS/MS Method to Study the Pharmacokinetic Interaction between Sorafenib and Dapagliflozin in Rats. Molecules. 2022; 27(19):6190. https://doi.org/10.3390/molecules27196190
Chicago/Turabian StyleHe, Xueru, Ying Li, Yinling Ma, Yuhao Fu, Xuejiao Xun, Yanjun Cui, and Zhanjun Dong. 2022. "Development of UPLC-MS/MS Method to Study the Pharmacokinetic Interaction between Sorafenib and Dapagliflozin in Rats" Molecules 27, no. 19: 6190. https://doi.org/10.3390/molecules27196190
APA StyleHe, X., Li, Y., Ma, Y., Fu, Y., Xun, X., Cui, Y., & Dong, Z. (2022). Development of UPLC-MS/MS Method to Study the Pharmacokinetic Interaction between Sorafenib and Dapagliflozin in Rats. Molecules, 27(19), 6190. https://doi.org/10.3390/molecules27196190






