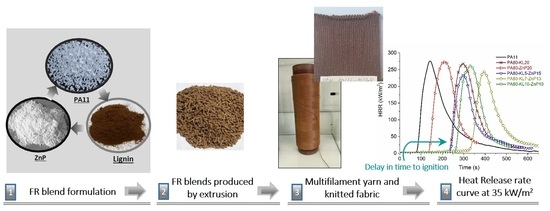
Development of Novel Polyamide 11 Multifilaments and Fabric Structures Based on Industrial Lignin and Zinc Phosphinate as Flame Retardants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blend Preparation and Processing
2.3. Multifilament Development via Melt Spinning
2.4. Knitted Fabric Development
2.5. Characterization Techniques
2.5.1. Melt Flow Index
2.5.2. Optical Microscopy
2.5.3. Mechanical Properties
2.5.4. Thermal Decomposition
2.5.5. Raman Spectroscopy
2.5.6. Fire Behavior
3. Results and Discussion
3.1. Melt Fluidity
3.2. Structure and Surface Properties
3.3. Mechanical Properties of Multifilament Yarns
3.4. Thermal Properties
3.5. Analysis of Released Products
3.6. Fire Behavior Analysis of Polymer Bulk
3.7. Fire Behavior Analysis of Fabric Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Darnerud, P.O. Toxic effects of brominated flame retardants in man and in wildlife. Environ. Int. 2003, 29, 841–853. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Guan, J.-P.; Tang, R.-C.; Liu, K.-Q. Phytic acid as a bio-based phosphorus flame retardant for poly(lactic acid) nonwoven fabric. J. Clean. Prod. 2016, 124, 114–119. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, Y.; Li, D.; He, S.; Zhang, F.; Zhang, G. A plant-based reactive ammonium phytate for use as a flame-retardant for cotton fabric. Carbohydr. Polym. 2017, 175, 636–644. [Google Scholar] [CrossRef]
- Morgan, A.B.; Wilkie, C.A. Non-Halogenated Flame Retardant Handbook; Scrivener Publishing: Beverly, MA, USA, 2014; ISBN 9781118939239. [Google Scholar]
- Li, B.; Zhang, X.; Su, R. An investigation of thermal degradation and charring of larch lignin in the condensed phase: The effects of boric acid, guanyl urea phosphate, ammonium dihydrogen phosphate and ammonium polyphosphate. Polym. Degrad. Stab. 2002, 75, 35–44. [Google Scholar] [CrossRef]
- Bertini, F.; Canetti, M.; Cacciamani, A.; Elegir, G.; Orlandi, M.; Zoia, L. Effect of ligno-derivatives on thermal properties and degradation behavior of poly(3-hydroxybutyrate)-based biocomposites. Polym. Degrad. Stab. 2012, 97, 1979–1987. [Google Scholar] [CrossRef]
- Canetti, M.; Bertini, F.; De Chirico, A.; Audisio, G. Thermal degradation behaviour of isotactic polypropylene blended with lignin. Polym. Degrad. Stab. 2006, 91, 494–498. [Google Scholar] [CrossRef]
- Song, P.; Cao, Z.; Fu, S.; Fang, Z.; Wu, Q.; Ye, J. Thermal degradation and flame retardancy properties of ABS/lignin: Effects of lignin content and reactive compatibilization. Thermochim. Acta 2011, 518, 59–65. [Google Scholar] [CrossRef]
- De Chirico, A.; Armanini, M.; Chini, P.; Cioccolo, G.; Provasoli, F.; Audisio, G. Flame retardants for polypropylene based on lignin. Polym. Degrad. Stab. 2003, 79, 139–145. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA including starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Lu, W.; Li, Q.; Zhang, Y.; Yu, H.; Hirose, S.; Hatakeyama, H.; Matsumoto, Y. Lignosulfonate/APP IFR and its flame retardancy in lignosulfonate-based rigid polyurethane foams. J. Wood Sci. 2018, 64, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Sonnier, R.; Dumazert, L. Influence of Ammonium Polyphosphate/Lignin Ratio on Thermal and Fire Behavior of Biobased Thermoplastic: The Case of Polyamide 11. Materials 2019, 12, 1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, C.K.; Li, Z.; Li, X.; Zhang, Z.; Hu, Y. Graphene oxide functionalized biomolecules for improved flame retardancy of Polyamide 66 fabrics with intact physical properties. Int. J. Biol. Macromol. 2020, 156, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Fierro, V.; Celzard, A. PLA with intumescent system containing lignin and ammonium polyphosphate for flame retardant textile. Polymers 2016, 8, 331. [Google Scholar] [CrossRef] [Green Version]
- Arkema Inc. Rilsan® PA11: Created from a Renewable Source. 2012. Available online: https://www.researchgate.net/file.PostFileLoader.html?id=56bf97be7eddd380108b458d&assetKey=AS%3A328772155920384%401455396798180 (accessed on 22 October 2020).
- Da Costa, A.P.; Botelho, E.C.; Costa, M.L.; Narita, N.E.; Tarpani, J.R. A Review of Welding Technologies for Thermoplastic Composites in Aerospace Applications. J. Aerosp. Technol. Manag. 2012, 4, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Holbery, J.; Houston, D. Natural-fiber-reinforced polymer composites in automotive applications. JOM 2006, 58, 80–86. [Google Scholar] [CrossRef]
- Cook, J. Handbook of Textile Fibres. Natural Fibres, 5th ed.; Woodhead Publishing: Cambridge, UK, 2004. [Google Scholar]
- Wesołowski, J.; Płachta, K. The Polyamide Market. Fibres Text. East. Eur. 2016, 24, 12–18. [Google Scholar] [CrossRef]
- Levchik, S.V.; Costa, L.; Camino, G. Effect of the fire-retardant, ammonium polyphosphate, on the thermal decomposition of aliphatic polyamides. I. Polyamides 11 and 12. Polym. Degrad. Stab. 1992, 36, 31–41. [Google Scholar] [CrossRef]
- Jin, X.; Chen, C.; Sun, J.; Zhang, X.; Gu, X.; Zhang, S. The synergism between melamine and expandable graphite on improving the flame retardancy of polyamide 11. High Perform. Polym. 2017, 29, 77–86. [Google Scholar] [CrossRef]
- Hao, A.; Wong, I.; Wu, H.; Lisco, B.; Ong, B.; Sallean, A.; Butler, S.; Londa, M.; Koo, J.H. Mechanical, thermal, and flame-retardant performance of polyamide 11–halloysite nanotube nanocomposites. J. Mater. Sci. 2015, 50, 157–167. [Google Scholar] [CrossRef]
- Lao, S.C.; Koo, J.H.; Moon, T.J.; Londa, M.; Ibeh, C.C.; Wissler, G.E.; Pilato, L.A. Flame-retardant polyamide 11 nanocomposites: Further thermal and flammability studies. J. Fire Sci. 2011, 29, 479–498. [Google Scholar] [CrossRef]
- Hou, W.; Fu, Y.; Zeng, C.; Liu, N.; Yin, C. Enhancement of flame retardancy and mechanical properties of polyamide 6 by incorporating melamine cyanurate combined with attapulgite. J. Appl. Polym. Sci. 2020, 137, 47298. [Google Scholar] [CrossRef]
- Lao, S.C.; Koo, J.H.; Moon, T.J.; Londa, M.; Ibeh, C.C.; Wissler, G.E.; Pilato, L.A. Flame-retardant Polyamide 11 and 12 nanocomposites: Thermal and Flammability Properties. J. Compos. Mater. 2009, 43, 1803–1818. [Google Scholar] [CrossRef]
- Bourbigot, S.; Flambard, X. Heat Resistance and Flammability of High Performance Fibres: A Review. Fire Mater. 2002, 26, 155–168. [Google Scholar] [CrossRef]
- Horrocks, R.; Sitpalan, A.; Zhou, C.; Kandola, B.K. Flame Retardant Polyamide Fibres: The Challenge of Minimising Flame Retardant Additive Contents with Added Nanoclays. Polymers 2016, 8, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weil, E.D.; Levchik, S.V. Flame Retardants for Plastics and Textiles; Hanser Hanser Publications: Munich, Germany, 2016; ISBN 9781569904541. [Google Scholar]
- Didane, N.; Giraud, S.; Devaux, E.; Lemort, G. A comparative study of POSS as synergists with zinc phosphinates for PET fire retardancy. Polym. Degrad. Stab. 2012, 97, 383–391. [Google Scholar] [CrossRef]
- Vasiljević, J.; Čolović, M.; Jerman, I.; Simončič, B.; Demšar, A.; Samaki, Y.; Šobak, M.; Šest, E.; Golja, B.; Leskovšek, M.; et al. In situ prepared polyamide 6/DOPO-derivative nanocomposite for melt-spinning of flame retardant textile filaments. Polym. Degrad. Stab. 2019, 166, 50–59. [Google Scholar] [CrossRef]
- Mandlekar, N.; Malucelli, G.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Guan, J. Fire retardant action of zinc phosphinate and polyamide 11 blend containing lignin as a carbon source. Polym. Degrad. Stab. 2018, 153, 63–74. [Google Scholar] [CrossRef]
- Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Guan, J. Valorization of Industrial Lignin as Biobased Carbon Source in Fire Retardant System for Polyamide 11 Blends. Polymers 2019, 11, 180. [Google Scholar] [CrossRef] [Green Version]
- ASTM. D1238-13 Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar] [CrossRef]
- ISO. ISO 5660-1:2002-Reaction-to-Fire Tests--Heat Release, Smoke Production and Mass Loss Rate--Part 1: Heat Release Rate (Cone Calorimeter Method); International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef] [Green Version]
- Bouajila, J.; Dole, P.; Joly, C.; Limare, A. Some laws of a lignin plasticization. J. Appl. Polym. Sci. 2006, 102, 1445–1451. [Google Scholar] [CrossRef]
- Sallem-Idrissi, N.; Van Velthem, P.; Sclavons, M. Fully Bio-Sourced Nylon 11/Raw Lignin Composites: Thermal and Mechanical Performances. J. Polym. Environ. 2018, 26, 4405–4414. [Google Scholar] [CrossRef]
- Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Malucelli, G.; Guan, J. Thermal Stability and Fire Retardant Properties of Polyamide 11 Microcomposites Containing Different Lignins. Ind. Eng. Chem. Res. 2017, 56, 13704–13714. [Google Scholar] [CrossRef]
- Nassar, M.M.; MacKay, G.D.M. Mechanism of thermal decomposition of lignin. Wood Fiber Sci. 1984, 16, 441–453. [Google Scholar]
- Braun, U.; Schartel, B. Flame Retardancy Mechanisms of Aluminium Phosphinate in Combination with Melamine Cyanurate in Glass-Fibre-Reinforced Poly(1,4-butylene terephthalate). Macromol. Mater. Eng. 2008, 293, 206–217. [Google Scholar] [CrossRef]
- Vannier, A.; Duquesne, S.; Bourbigot, S.; Alongi, J.; Camino, G.; Delobel, R. Investigation of the thermal degradation of PET, zinc phosphinate, OMPOSS and their blends—Identification of the formed species. Thermochim. Acta 2009, 495, 155–166. [Google Scholar] [CrossRef]
- Shukla, A.; Sharma, V.; Basak, S.; Ali, S.W. Sodium lignin sulfonate: A bio-macromolecule for making fire retardant cotton fabric. Cellulose 2019, 26, 8191–8208. [Google Scholar] [CrossRef]
- Mailhos-Lefievre, V.; Sallet, D.; Martel, B. Thermal degradation of pure and flame-retarded polyamides 11 and 12. Polym. Degrad. Stab. 1989, 23, 327–336. [Google Scholar] [CrossRef]
- Braun, U.; Bahr, H.; Sturm, H.; Schartel, B. Flame retardancy mechanisms of metal phosphinates in combination with melamine cyanurate in glass-fiber reinforced poly (1,4-butylene terephthalate): The influence of metal cation. Polym. Adv. Technol. 2008, 19, 680–692. [Google Scholar] [CrossRef]
- Brebu, M.; Tamminen, T.; Spiridon, I. Thermal degradation of various lignins by TG-MS/FTIR and Py-GC-MS. J. Anal. Appl. Pyrolysis 2013, 104, 531–539. [Google Scholar] [CrossRef]
- Levchik, S.V.; Costa, L.; Camino, G. Effect of the fire-retardant ammonium polyphosphate on the thermal decomposition of aliphatic polyamides. Part III-Polyamides 6.6 and 6.10. Polym. Degrad. Stab. 1994, 43, 43–54. [Google Scholar] [CrossRef]
- Naik, A.D.; Fontaine, G.; Samyn, F.; Delva, X.; Louisy, J.; Bellayer, S.; Bourgeois, Y.; Bourbigot, S. Outlining the mechanism of flame retardancy in polyamide 66 blended with melamine-poly(zinc phosphate). Fire Saf. J. 2014, 70, 46–60. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies Contents, 3rd ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2001; ISBN 0471852988. [Google Scholar]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Mouritz, A.P.; Mathys, Z.; Gibson, A.G. Heat release of polymer composites in fire. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1040–1054. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Ferry, L.; Dorez, G.; Taguet, A.; Otazaghine, B.; Lopez-Cuesta, J.M. Chemical modification of lignin by phosphorus molecules to improve the fire behavior of polybutylene succinate. Polym. Degrad. Stab. 2015, 113, 135–143. [Google Scholar] [CrossRef]
- Bacsa, W.S.; Ugarte, D.; Châtelain, A.; de Heer, W.A. High-resolution electron microscopy and inelastic light scattering of purified multishelled carbon nanotubes. Phys. Rev. B 1994, 50, 15473–15476. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, B.; Zhang, X.; Su, R. The study of flame retardants on thermal degradation and charring process of manchurian ash lignin in the condensed phase. Polym. Degrad. Stab. 2001, 72, 493–498. [Google Scholar] [CrossRef]
- Liu, L.; Huang, G.; Song, P.; Yu, Y.; Fu, S. Converting Industrial Alkali Lignin to Biobased Functional Additives for Improving Fire Behavior and Smoke Suppression of Polybutylene Succinate. ACS Sustain. Chem. Eng. 2016, 4, 4732–4742. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Novel flame retardant finishing systems for cotton fabrics based on phosphorus-containing compounds and silica derived from sol–gel processes. Carbohydr. Polym. 2011, 85, 599–608. [Google Scholar] [CrossRef]










| Sample | Elastic Modulus (MPa) | Force at Break (cN) | Elongation (%) | Tenacity (cN/Tex) |
|---|---|---|---|---|
| PA11 | 1.22 ± 0.20 | 75 ± 11 | 194 ± 32 | 21.2 ± 2.3 |
| PA80-KL5-ZnP15 | 1.28 ± 0.20 | 77 ± 5 | 173 ± 15 | 19.8 ± 1.7 |
| PA80-KL7-ZnP13 | 1.12 ± 0.13 | 57 ± 9 | 158 ± 15 | 16.1 ± 1.5 |
| PA80-KL10-ZnP10 | 1.68 ± 0.14 | 64 ± 7 | 122 ± 13 | 18.5 ± 1.5 |
| PA80-LL5-ZnP15 | 0.57 ± 0.20 | 47 ± 6 | 253 ± 37 | 12.7 ± 2.2 |
| PA80-LL7-ZnP13 | 0.81 ± 0.20 | 51 ± 6 | 178 ± 61 | 12.4 ± 1.7 |
| PA80-LL10-ZnP10 | 0.60 ± 0.29 | 39 ± 8 | 246 ± 65 | 12.0 ± 4.2 |
| Sample | T5% (°C) | Tmax (°C) | MMLR (%/°C) | Rexp@700 (%) | Rcal (%) |
|---|---|---|---|---|---|
| PA11 | 396 | 423 | 2.0 | 0.7 | - |
| PA80-LL20 | 285 | 468 | 1.6 | 13.5 | 12.5 |
| PA80-KL20 | 341 | 435 | 1.4 | 12.4 | 9.1 |
| PA80-ZnP20 | 366 | 473 | 2.3 | 1.2 | 4.8 |
| PA80-LL5-ZnP15 | 373 | 472 | 2.4 | 5.8 | 6.7 |
| PA80-LL7-ZnP13 | 364 | 467 | 2.0 | 8.2 | 7.5 |
| PA80-LL10-ZnP10 | 326 | 465 | 1.8 | 12.7 | 8.6 |
| PA80-KL5-ZnP15 | 359 | 473 | 2.0 | 5.4 | 5.8 |
| PA80-KL7-ZnP13 | 347 | 472 | 1.8 | 6.4 | 6.3 |
| PA80-KL10-ZnP10 | 339 | 453 | 1.6 | 8.9 | 6.9 |
| Decomposition Products | Functional Group | Characteristic Peak (cm−1) |
|---|---|---|
| Polyamide 11 | ||
| Hydrocarbon species (R-CH2-CH3) | -C-H2- | 2930, 2856, 1460, 723 |
| Alkyl vinyl (R-CH=CH2) | -CH=CH2 (cis-substitution) | 1460, 965, 913 |
| Olefin (conjugated) | -C=C- | 1497 |
| Carbonyl compound | >C=O | 1706 |
| Alkyl nitrile | -C≡N | 2245 |
| Amide compound | -CO-NH2- | 1683 |
| PA80-KL10-ZnP10 | ||
| Phosphinate compound | P=O, P-O | 1140, 1058, 772 |
| Phosphinic acid | P-OH | 3650, 1270, 855 |
| Hydrocarbon species (-CH2-, CH3-) | C-H | 2930, 2856, 1460, 723 |
| Phenolic compound | -C=C-, -OH | 1498, 3340 |
| Carbonyl compound | C=O | 1706, 1675 (conjugation) |
| Carbon dioxide | O=C=O | 2298, 668 |
| PA80-LL10-ZnP10 | ||
| Phosphinate compound | P=O, P-O | 1131, 1056, 772 |
| Hydrocarbon species (-CH2-, CH3-) | C-H | 2930, 2856, 1460, 723 |
| Phosphinic acid | P-OH | 3650, 1270, 855 |
| Carbonyl compound | C=O | 1706 |
| Phenolic compound | -C=C-, -OH | 1496, 3340 |
| Carbon dioxide | O=C=O | 2298, 668 |
| Sample | TTI (s) | ∆ (%) | PHRR (kW/m2) | ∆ (%) | THR (MJ/m2) | ∆ (%) | MARHE (kW/m2) | ∆ (%) |
|---|---|---|---|---|---|---|---|---|
| PA11 | 154 ± 3 | - | 884 ± 4 | - | 92 ± 4 | - | 281 ± 2 | - |
| PA80-LL20 | 72 ± 12 | −53 | 454 ± 30 | −49 | 78 ± 6 | −15 | 218 ± 5 | −22 |
| PA80-KL20 | 112 ± 10 | −27 | 821 ± 27 | −7 | 90 ± 2 | −2 | 284 ± 3 | +1 |
| PA80-ZnP20 | 223 ± 14 | +31 | 825 ± 29 | −7 | 88 ± 6 | −4 | 220 ± 4 | −22 |
| PA80-LL5-ZnP15 | 112 ± 12 | −27 | 560 ± 40 | −37 | 79 ± 2 | −14 | 192 ± 20 | −32 |
| PA80-LL7-ZnP13 | 92 ± 9 | −40 | 443 ± 21 | −50 | 77 ± 4 | −16 | 167 ± 7 | −41 |
| PA80-LL10-ZnP10 | 86 ± 9 | −44 | 315 ± 11 | −64 | 73 ± 2 | −21 | 143 ± 14 | −49 |
| PA80-KL5-ZnP15 | 150 ± 18 | −3 | 740 ± 23 | −16 | 79 ± 2 | −14 | 163 ± 11 | −42 |
| PA80-KL7-ZnP13 | 128 ± 14 | −17 | 678 ± 36 | −23 | 77 ± 3 | −16 | 148 ± 15 | −47 |
| PA80-KL10-ZnP10 | 116 ± 13 | −25 | 500 ± 48 | −43 | 75 ± 7 | −18 | 127 ± 18 | −55 |
| Sample | TTI (s) | ∆ (%) | PHRR (kW/m2) | ∆ (%) | THR (MJ/m2) | ∆ (%) | MARHE (kW/m2) | ∆ (%) |
|---|---|---|---|---|---|---|---|---|
| PA11 | 78 ± 5 | - | 278 ± 2 | - | 38 ± 4 | - | 114 ± 4 | - |
| PA80-KL20 | 184 ± 68 | +58 | 246 ± 30 | −12 | 31 ± 4 | −17 | 75 ± 3 | −34 |
| PA80-ZnP20 | 122 ± 21 | +36 | 287 ± 19 | +3 | 32 ± 2 | −15 | 106 ± 5 | −7 |
| PA80-KL5-ZnP15 | 209 ± 40 | +63 | 234 ± 1 | −16 | 30 ± 1 | −21 | 66 ± 7 | −43 |
| PA80-KL7-ZnP13 | 294 ± 42 | +73 | 237 ± 8 | −15 | 32 ± 4 | −16 | 52 ± 0 | −54 |
| PA80-KL10-ZnP10 | 198 ± 90 | +61 | 247 ± 20 | −11 | 31 ± 0 | −17 | 75 ± 7 | −34 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Guan, J. Development of Novel Polyamide 11 Multifilaments and Fabric Structures Based on Industrial Lignin and Zinc Phosphinate as Flame Retardants. Molecules 2020, 25, 4963. https://doi.org/10.3390/molecules25214963
Mandlekar N, Cayla A, Rault F, Giraud S, Salaün F, Guan J. Development of Novel Polyamide 11 Multifilaments and Fabric Structures Based on Industrial Lignin and Zinc Phosphinate as Flame Retardants. Molecules. 2020; 25(21):4963. https://doi.org/10.3390/molecules25214963
Chicago/Turabian StyleMandlekar, Neeraj, Aurélie Cayla, François Rault, Stéphane Giraud, Fabien Salaün, and Jinping Guan. 2020. "Development of Novel Polyamide 11 Multifilaments and Fabric Structures Based on Industrial Lignin and Zinc Phosphinate as Flame Retardants" Molecules 25, no. 21: 4963. https://doi.org/10.3390/molecules25214963
APA StyleMandlekar, N., Cayla, A., Rault, F., Giraud, S., Salaün, F., & Guan, J. (2020). Development of Novel Polyamide 11 Multifilaments and Fabric Structures Based on Industrial Lignin and Zinc Phosphinate as Flame Retardants. Molecules, 25(21), 4963. https://doi.org/10.3390/molecules25214963