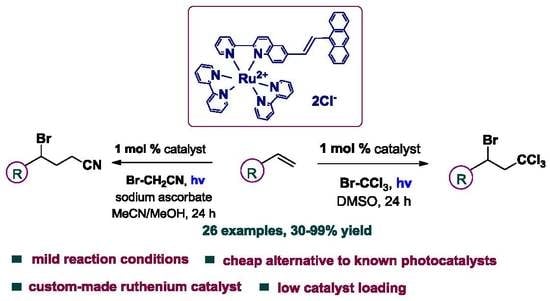
Photocatalytic Atom Transfer Radical Addition to Olefins Utilizing Novel Photocatalysts
Abstract
:1. Introduction
2. Results
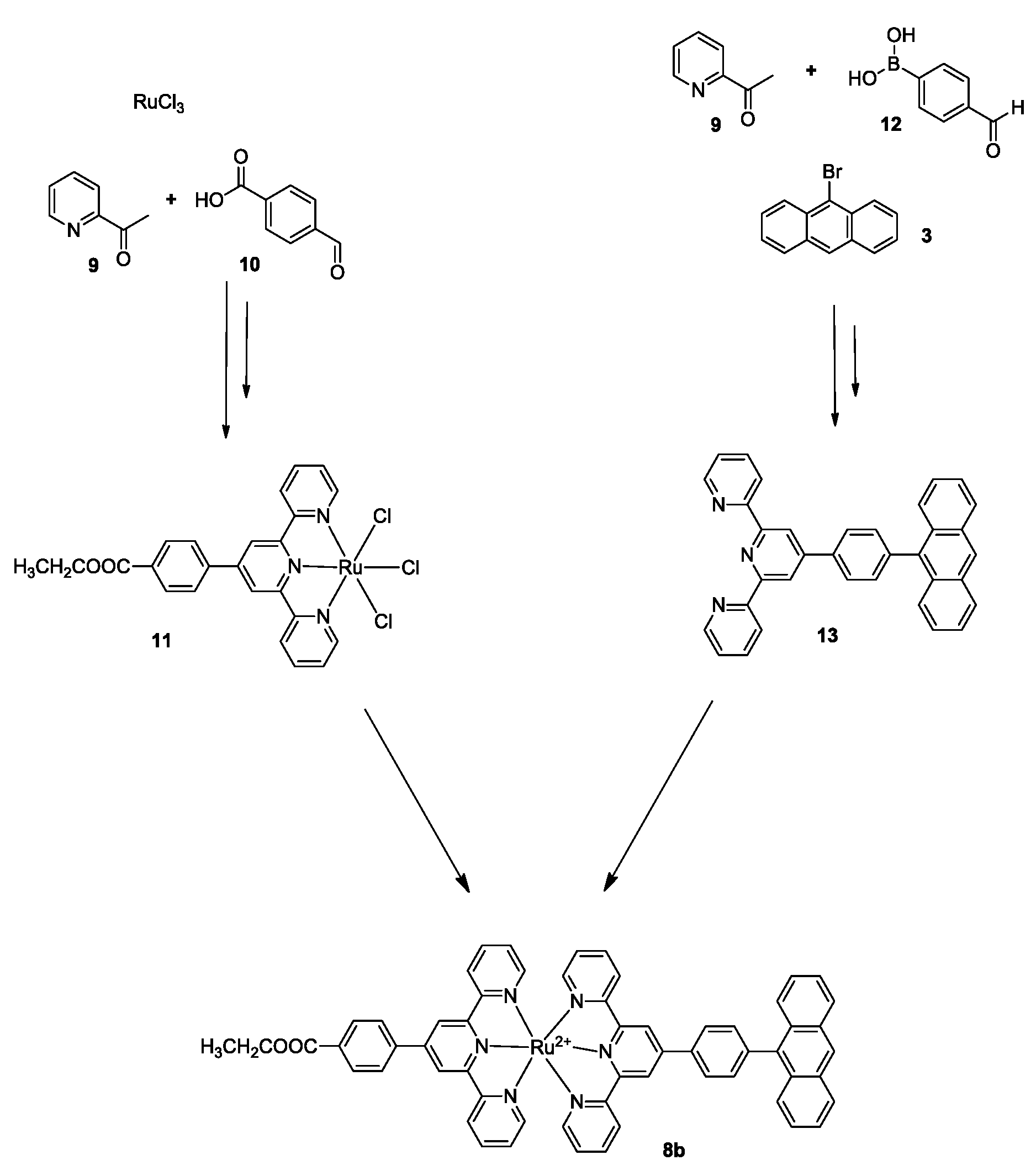
2.1. Synthesis of Photocatalysts
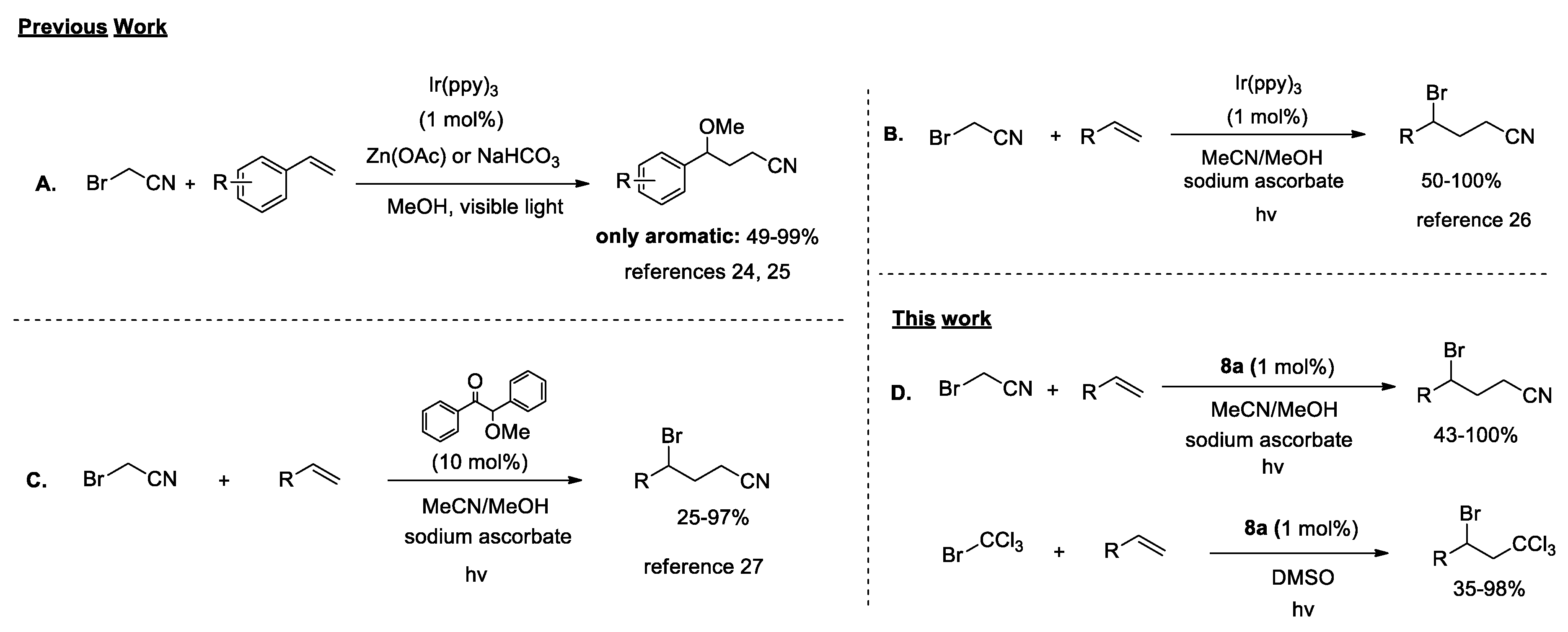
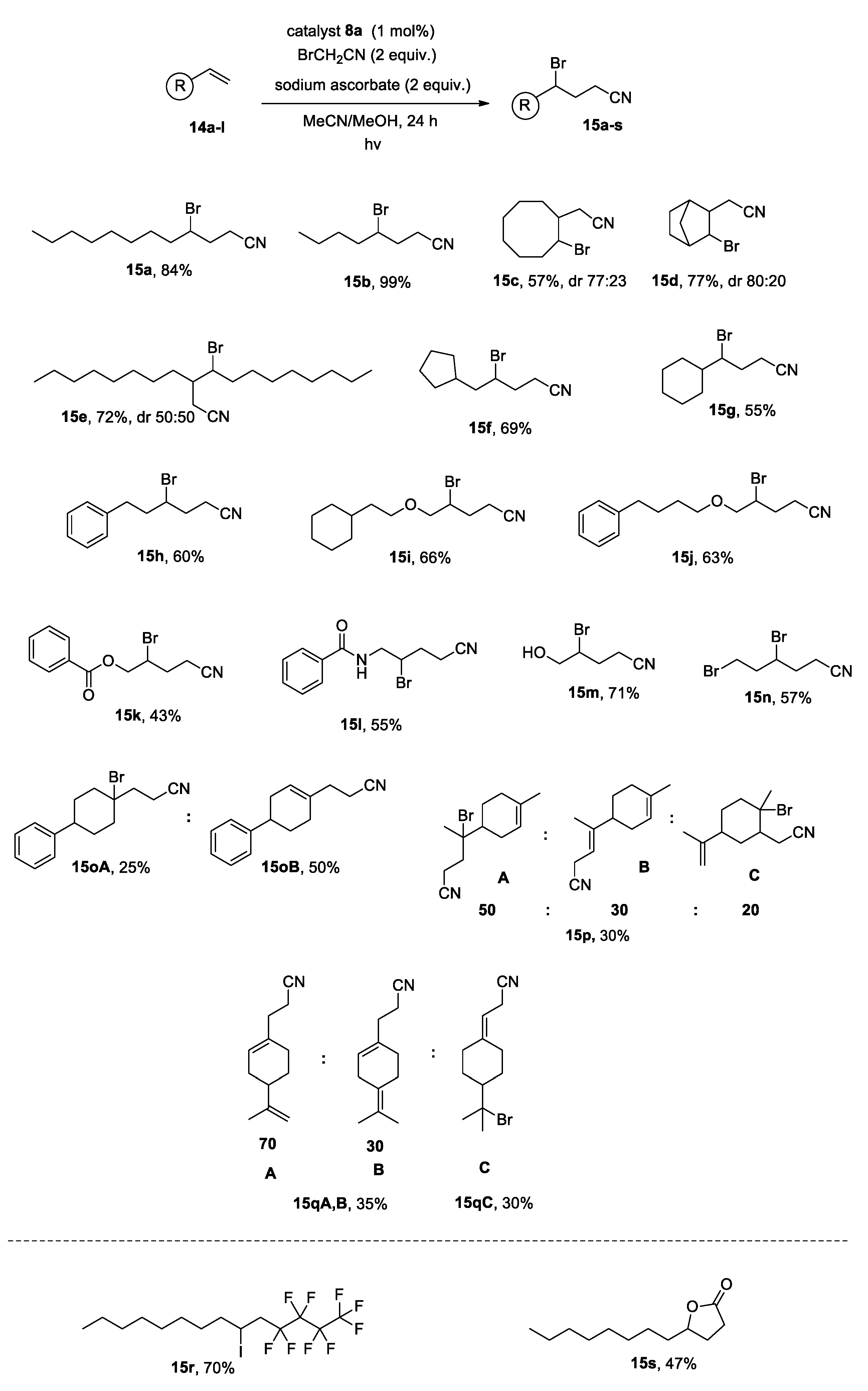
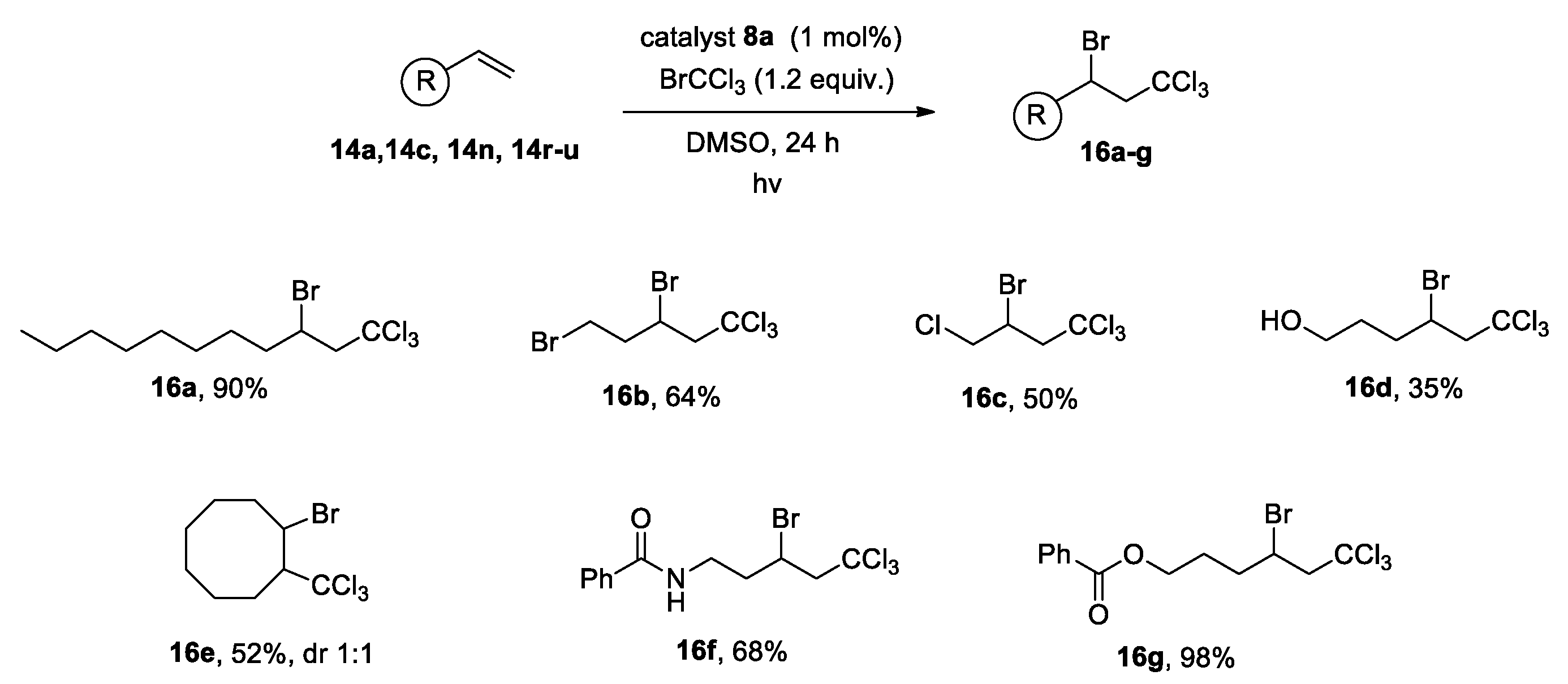
2.2. Photocatalytic Reactions
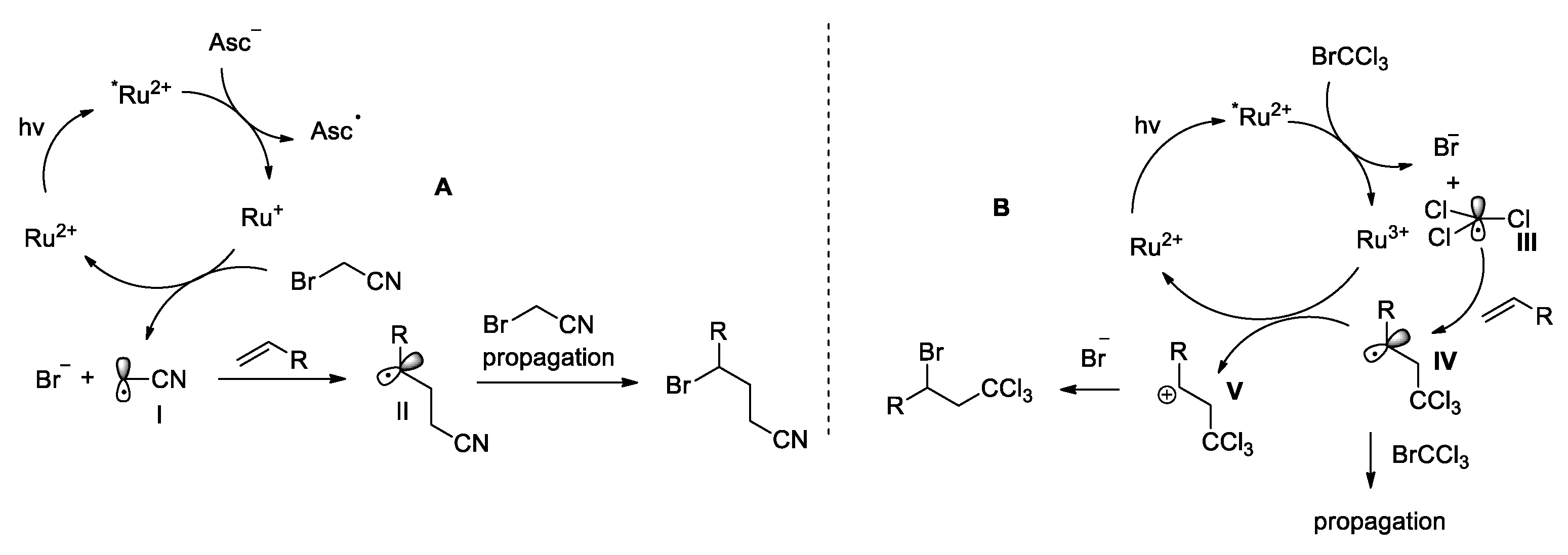
2.3. Mechanistic Studies
2.3.1. Phosphorescence Quenching Studies
2.3.2. Quantum Yield Measurement
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ciamician, G. The photochemistry of the future. Science 1912, 36, 385–394. [Google Scholar] [CrossRef]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Ischay, M.A.; Anzovino, M.E.; Du, J.; Yoon, T.P. Efficient visible light photocatalysis of [2+2] enone cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. [Google Scholar] [CrossRef] [PubMed]
- Narayanam, J.M.R.; Tuckler, J.W.; Stephenson, C.R.J. Electron-transfer photoredox catalysis: Development of a tin-free reductive dehalogenation reaction. J. Am. Chem. Soc. 2009, 131, 8756–8757. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nature Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.W.; Stephenson, C.R.J.J. Shining Light on Photoredox Catalysis: Theory and Synthetic Applications. Org. Chem. 2012, 77, 1617–1622. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; Macmillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Skubi, K.L.; Blum, T.R.; Yoon, T.P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 2016, 116, 10035–10074. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef]
- Karkas, M.D.; Porco, J.A., Jr.; Stephenson, C.R.J. Photochemical aproaches to complex chemotypes: Applications in natural product synthesis. Chem. Rev. 2016, 116, 9683–9747. [Google Scholar] [CrossRef]
- Ravelli, D.; Protti, S.; Fagnoni, M. Carbon-carbon bond forming reactions via photogenerated. Chem. Rev. 2016, 116, 9580–9913. [Google Scholar] [CrossRef]
- Cambie, D.; Bottechia, C.; Straathof, N.J.W.; Hessel, V.; Noel, T. Applications of continuous-flow photochemistry in organic synthesis, material science, and water treatment. Chem. Rev. 2016, 116, 10276–10341. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, P.; Ji, Z.; Tang, G.; Zhao, Y. Copper-catalyzed cascade radical addition-cyclization halogen atom transfer between alkynes and unsaturated a-halogenocarbonyls. ACS Catal. 2017, 7, 186–190. [Google Scholar] [CrossRef]
- Severin, K. Ruthenium-catalyzed atom transfer radical addition reactions. Chimia 2012, 66, 386–388. [Google Scholar] [CrossRef]
- Nguyen, J.D.; Tucker, J.W.; Konieczynska, M.D.; Stephenson, C.R.J. Intermolecular atom transfer radical addition to olefins mediated by oxidative quenching of photoredox catalysts. J. Am. Chem. Soc. 2011, 133, 4160–4163. [Google Scholar] [CrossRef]
- Monks, B.M.; Cook, S.P. Palladium-catalyzed intramolecular iodine-transfer reactions in the presence of β-hydrogen atoms. Angew. Chem. Int. Ed. 2013, 52, 14214–14218. [Google Scholar] [CrossRef]
- Boivin, J.; Yousfi, M.; Zard, S.Z. A versatile radical based synthesis of γ-lactams using nickel powder / acetic acid. Tetrahedron Lett. 1994, 35, 5629–5632. [Google Scholar] [CrossRef]
- Couvart, T.; Masson, G. Recent Progress in Visible-Light Photoredox-Catalyzed Intermolecular 1.2-Difunctionalization of Double Bonds via an ATRA-Type Mechanism. J. Org. Chem. 2016, 81, 6945–6952. [Google Scholar]
- Brimioulle, R.; Bach, T. Enantioselective lewis acid catalysis of intramolecular enone [2+2] photocycloaddition reactions. Science 2013, 342, 840–843. [Google Scholar] [CrossRef]
- Romero, N.; Margrey, K.; Tay, N.; Nicewicz, D. Site-selective arene C-H amination via photoredox catalysis. Science 2015, 349, 1326–1330. [Google Scholar] [CrossRef]
- Silvi, M.; Verrier, C.; Rey, Y.P.; Buzzetti, L.; Melchiorre, P. Visible-light excitation of iminium ions enables the enantioselective catalytic β-alkylation of enals. Nature Chem. 2017, 9, 868–873. [Google Scholar] [CrossRef]
- Yorimitsu, H.; Shinokubo, H.; Matsubata, S.; Oshima, K. Triethylborane-induced bromine atom-transfer radical addition in aqueous media: Study of the solvent effect on radical addition reactions. J. Org. Chem. 2001, 66, 7776–7785. [Google Scholar] [CrossRef]
- Arceo, E.; Montroni, E.; Melchiorre, P. Photo-organocatalysis of atom-transfer radical additions to alkenes. Angew. Chem. Int. Ed. 2014, 53, 12064–12068. [Google Scholar] [CrossRef]
- Yi, H.; Zhang, X.; Qin, C.; Liao, Z.; Liu, J.; Lei, A. Visible light-induced γ-alkoxynitrile synthesis via three-component alkoxycyanomethylation of alkenes. Adv. Synth. Catal. 2014, 356, 2873–2877. [Google Scholar] [CrossRef]
- Fumagalli, G.; Boyd, S.; Greaney, M.F. Exploiting photoredox catalysis for the synthesis of tetra- and di-hydrofurans. Tetrahedron Lett. 2015, 56, 2571–2573. [Google Scholar] [CrossRef] [Green Version]
- Voutyritsa, E.; Triandafillidi, I.; Kokotos, C.G. Expanding the scope of photocatalysis: Atom transfer radical addition of bromoacetonitrile to aliphatic olefins. ChemCatChem 2018, 10, 2466–2470. [Google Scholar] [CrossRef]
- Voutyritsa, E.; Nikitas, N.F.; Apostolopoulou, M.K.; Gerogiannopoulou, A.D.D.; Kokotos, C.G. Photoorganocatalytic atom transfer radical addition of bromoacetonitrile to aliphatic olefins. Synthesis 2018, 50, 3395–3401. [Google Scholar]
- Bellesia, F.; Forti, L.; Ghelfi, F.; Pagnoni, U.M. The Fe0 promoted addition of CCl4, and CCl3Br to Olefins. Synth. Commun. 1997, 27, 961–971. [Google Scholar] [CrossRef]
- Sawama, Y.; Nakatani, R.; Imanishi, T.; Fujiwara, Y.; Monguchi, Y.; Sajiki, H. Effect of sodium acetate in atom transfer radical addition of polyhaloalkanes to olefins. RSC Adv. 2014, 4, 8657–8660. [Google Scholar] [CrossRef]
- Magagnano, G.; Gualandi, A.; Marchini, M.; Mengozzi, L.; Ceroni, P.; Cozzi, P.G. Photocatalytic ATRA reaction promoted by iodo-Bodipy and sodium ascorbate. Chem. Comm. 2017, 53, 1591–1594. [Google Scholar] [CrossRef]
- Kaplaneris, N.; Bisticha, A.; Papadopoulos, G.N.; Limnios, D.; Kokotos, C.G. Photoorganocatalytic synthesis of lactones via a selective C-H activation-alkylation of alcohols. Green Chem. 2017, 19, 4451–4456. [Google Scholar] [CrossRef]
- Triandafillidi, I.; Kokotou, M.G.; Kokotos, C.G. Photocatalytic synthesis of γ-lactones from alkenes: High-resolution mass spectrometry as a tool to study photoredox reactions. Org. Lett. 2018, 20, 36–39. [Google Scholar] [CrossRef]
- Papadopoulos, G.N.; Voutyritsa, E.; Kaplaneris, N.; Kokotos, C.G. Green photo-organocatalytic C-H activation of aldehydes: Selective hydroacylation of electron-defficient alkenes. Chem. Eur. J. 2018, 24, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Nikitas, N.F.; Triandafillidi, I.; Kokotos, C.G. Photo-organocatalytic synthesis of acetals from aldehydes. Green Chem. 2019, 21, 669–674. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Stergiopoulos, T.; Kantonis, G.; Kontos, A.G.; Papadopoulos, K.; Stublla, A.; Potvin, P.G.; Falaras, P. Terpyridine- and 2,6-dipyrazinylpyridine-coordinated ruthenium(II) complexes: Synthesis, characterization and application in TiO2-based dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2010, 214, 22–32. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Stergiopoulos, T.; Kontos, A.G.; Pefkianakis, E.K.; Papadopoulos, K.; Falaras, P. Novel Ru(II) sensitizers bearing an unsymmetrical pyridine-quinoline hybrid ligand with extended π-conjugation: Synthesis and application in dye-sensitized solar cells. Dalton Trans. 2013, 42, 6582–6591. [Google Scholar] [CrossRef]
- Pefkianakis, E.K.; Christodouleas, D.; Giokas, D.L.; Papadopoulos, K.; Vougioukalakis, G.C. A new family of Ru(II) photosensitizers with high singlet oxygen quantum yield: Synthesis, characterization, and evaluation. Eur. J. Inorg. Chem. 2013, 2013, 4628–4635. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Konstantakou, M.; Pefkianakis, E.K.; Kabanakis, A.N.; Stergiopoulos, T.; Kontos, A.G.; Andreopoulou, A.K.; Kallitsis, J.K.; Falaras, P. A novel ruthenium-based light-harvesting antenna bearing an anthracene moiety in dye-sensitized solar cells. Asian J. Org. Chem. 2014, 3, 953–962. [Google Scholar] [CrossRef]
- Pefkianakis, E.K.; Theodossiou, T.A.; Toubanaki, D.K.; Karagouni, E.; Falaras, P.; Papadopoulos, K.; Vougioukalakis, G.C. A family of potent Ru(II) photosensitizers with enhanced DNA intercalation: Bimodal photokillers. Photochem. Photobiol. 2015, 91, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, C.J.; Nguyen, J.D.; Finkbeiner, P.; Stephenson, C.R.J. Visible light-mediated atom transfer radical addition via oxidative and reductive quenching of photocatalysts. J. Am. Chem. Soc. 2012, 134, 8875–8884. [Google Scholar] [CrossRef]
- Cismenia, M.A.; Yoon, T.P. Characterizing chain processes in visible light photoredox catalysis. Chem. Sci. 2015, 6, 5426–5434. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



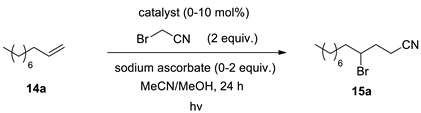
| Entry | Catalyst | Catalyst Loading (mol %) | Sodium Ascorbate (Equiv.) | Yield (%) a |
|---|---|---|---|---|
| 1 | Ir(ppy)3 (8c) | 1 | 2 | 98 |
| 2 | Ru(bpy)3Cl2 (8d) | 1 | 2 | 78 |
| 3 | 8a | 1 | 2 | 84 |
| 4 | 8b | 1 | 2 | 63 |
| 5 | 8a | 1 | - | 0 |
| 6 b | 8a | 1 | 2 | 0 |
| 7 | - | - | 2 | 0 |
| 8 | Thioxanthone (8e) | 10 | 2 | 43 |
| 9 | Eosin Y (8f) | 10 | 2 | 32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voutyritsa, E.; Triandafillidi, I.; Tzouras, N.V.; Nikitas, N.F.; Pefkianakis, E.K.; Vougioukalakis, G.C.; Kokotos, C.G. Photocatalytic Atom Transfer Radical Addition to Olefins Utilizing Novel Photocatalysts. Molecules 2019, 24, 1644. https://doi.org/10.3390/molecules24091644
Voutyritsa E, Triandafillidi I, Tzouras NV, Nikitas NF, Pefkianakis EK, Vougioukalakis GC, Kokotos CG. Photocatalytic Atom Transfer Radical Addition to Olefins Utilizing Novel Photocatalysts. Molecules. 2019; 24(9):1644. https://doi.org/10.3390/molecules24091644
Chicago/Turabian StyleVoutyritsa, Errika, Ierasia Triandafillidi, Nikolaos V. Tzouras, Nikolaos F. Nikitas, Eleftherios K. Pefkianakis, Georgios C. Vougioukalakis, and Christoforos G. Kokotos. 2019. "Photocatalytic Atom Transfer Radical Addition to Olefins Utilizing Novel Photocatalysts" Molecules 24, no. 9: 1644. https://doi.org/10.3390/molecules24091644