Photocatalytic Difluoromethylation Reactions of Aromatic Compounds and Aliphatic Multiple C–C Bonds
Abstract
:1. Introduction
2. Photocatalytic Difluoromethylation of (Hetero)aromatic Compounds
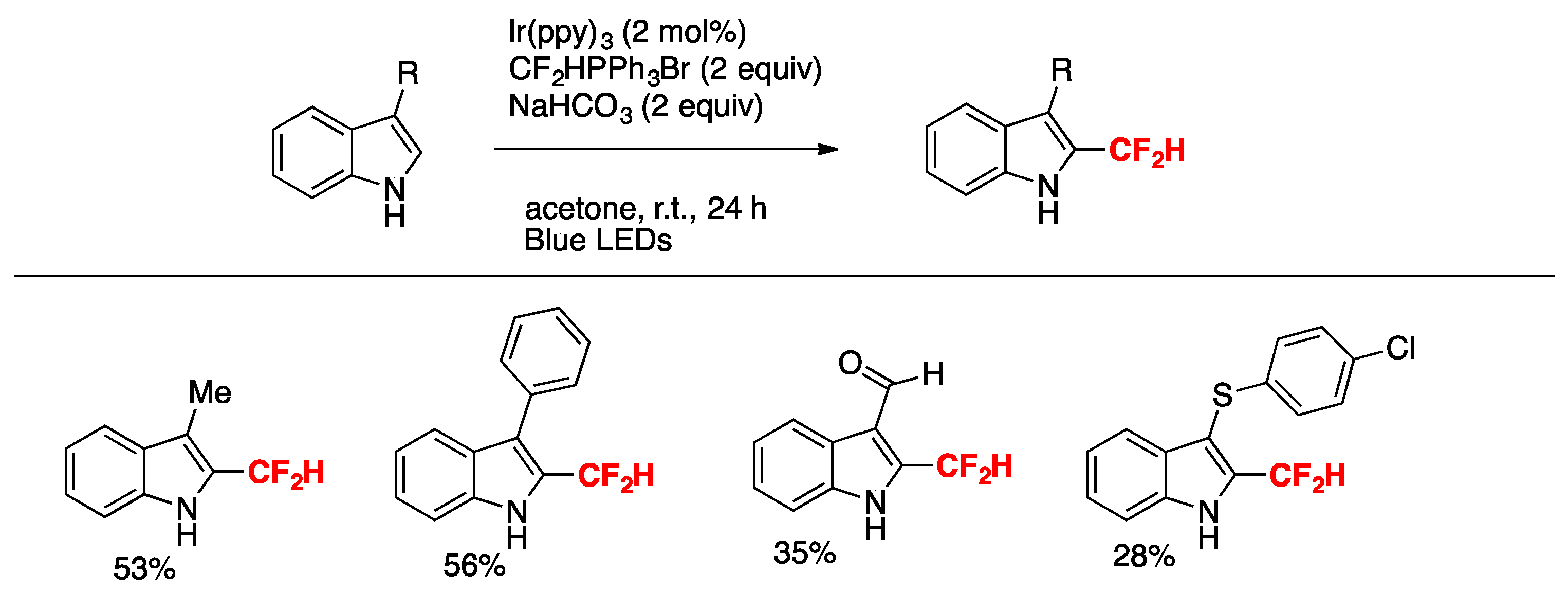
2.1. C(Het)-H Difluoromethylation of (Hetero)aromatic Compounds
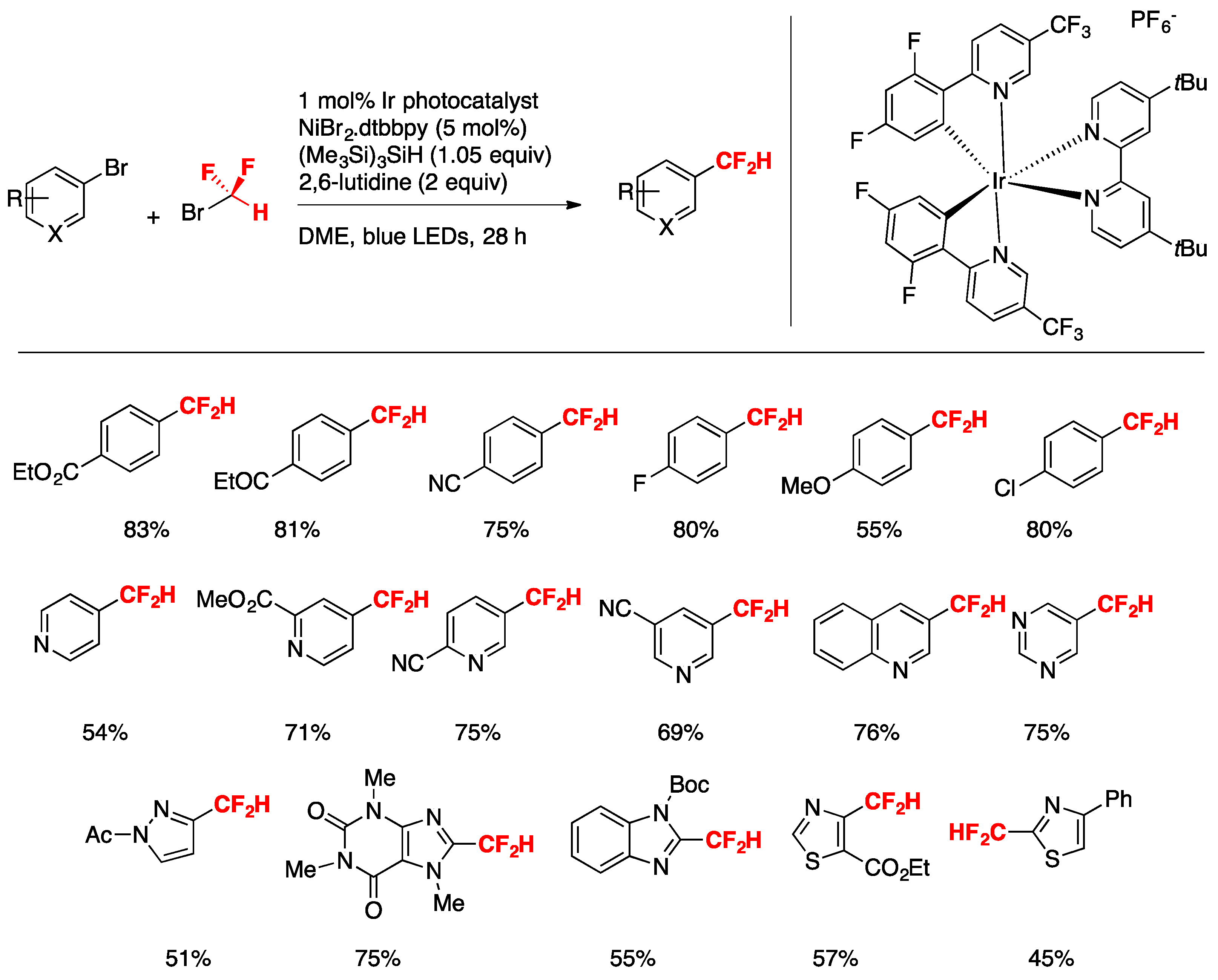
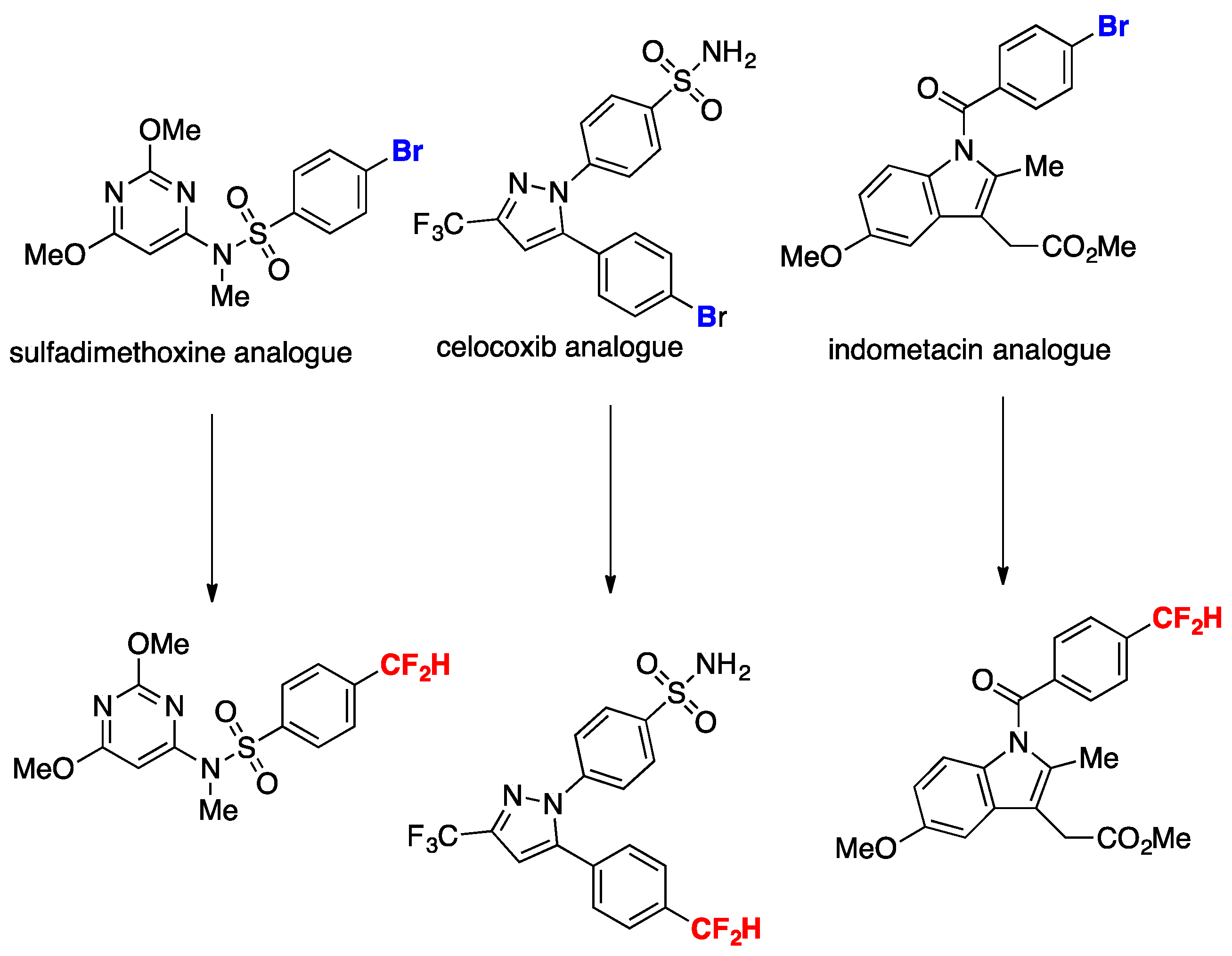
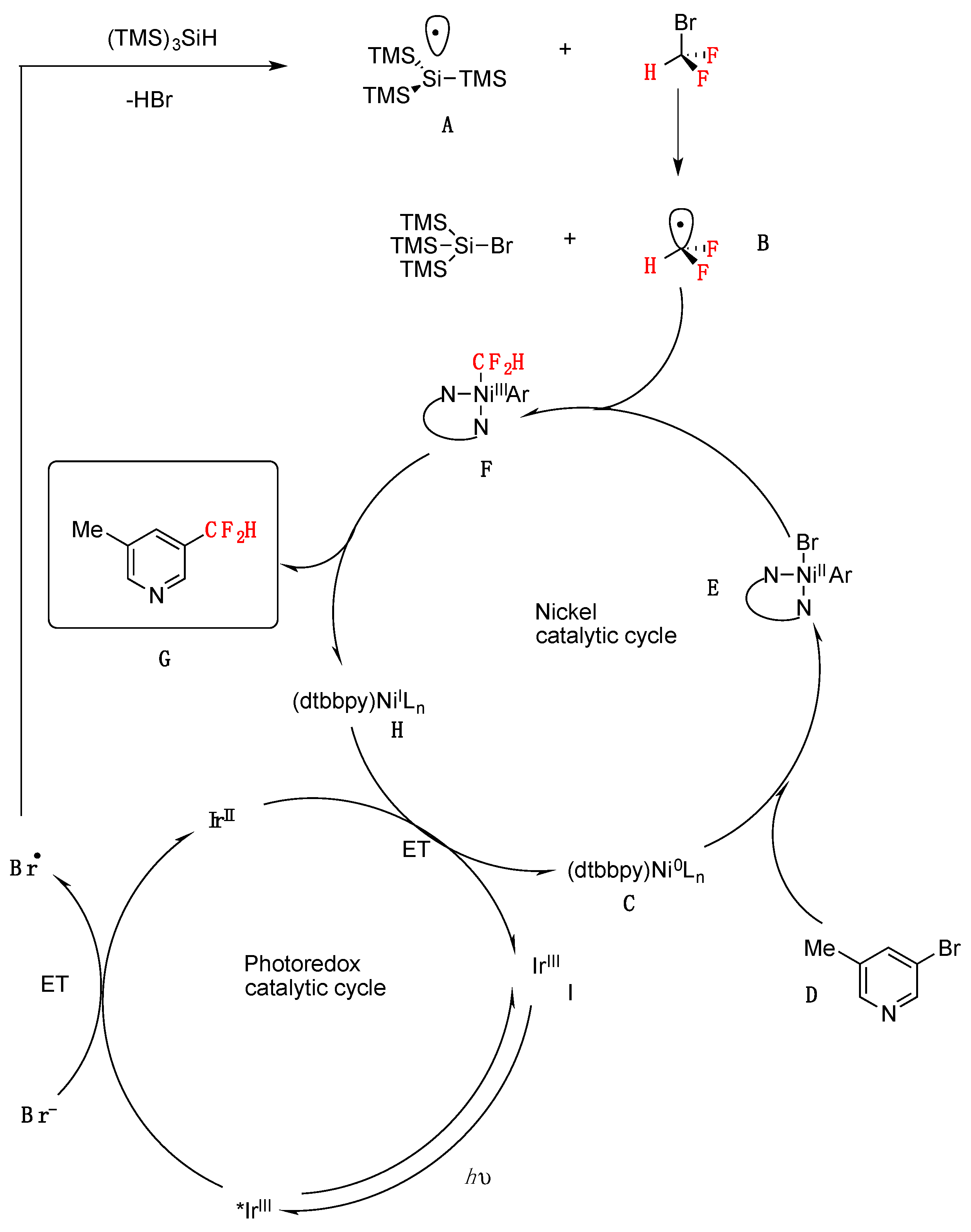
2.2. CF2H Ipso Substitution of (Hetero)aromatic Compounds
3. Photocatalytic Difluoromethylation of Multiple Bonds
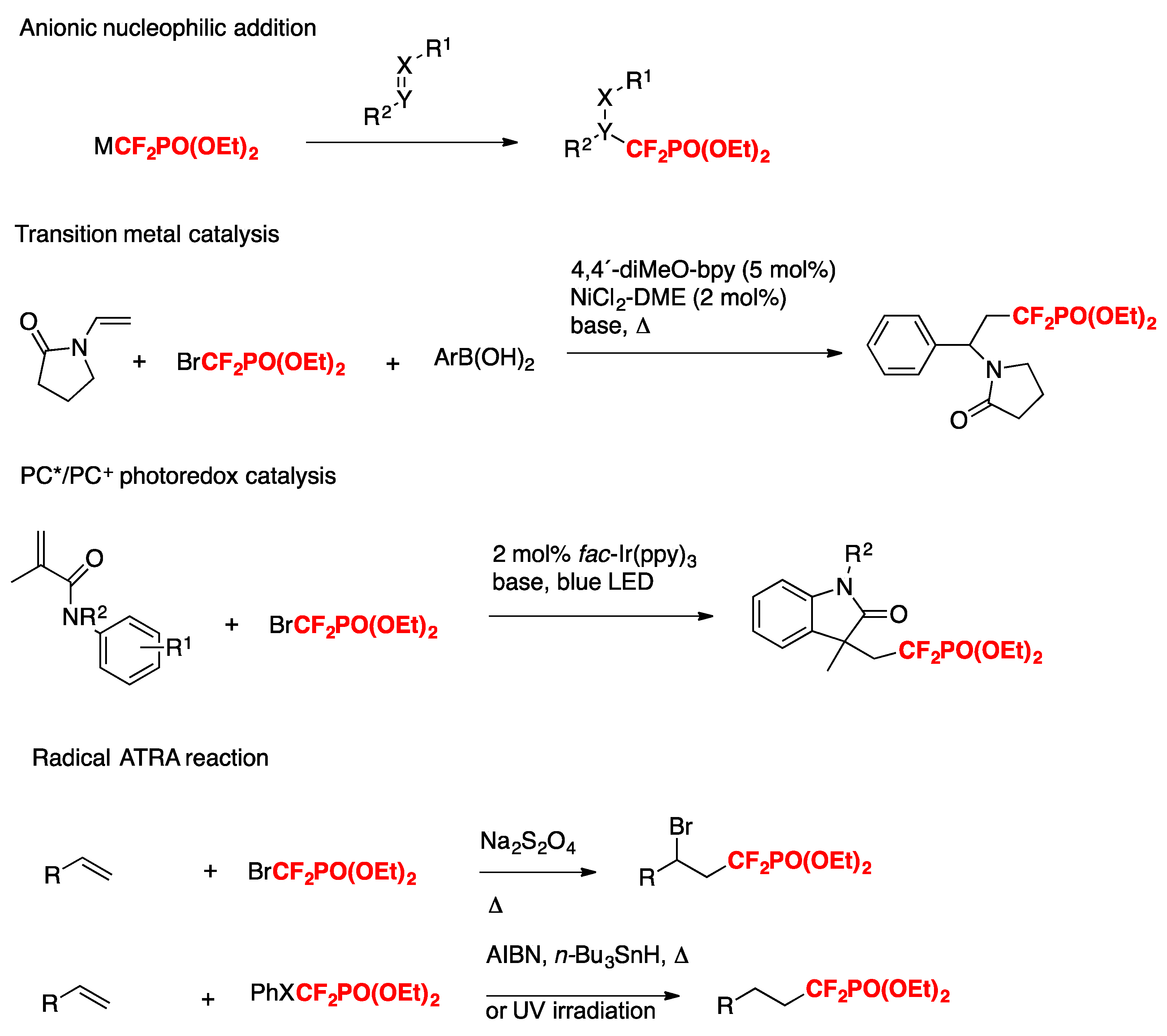
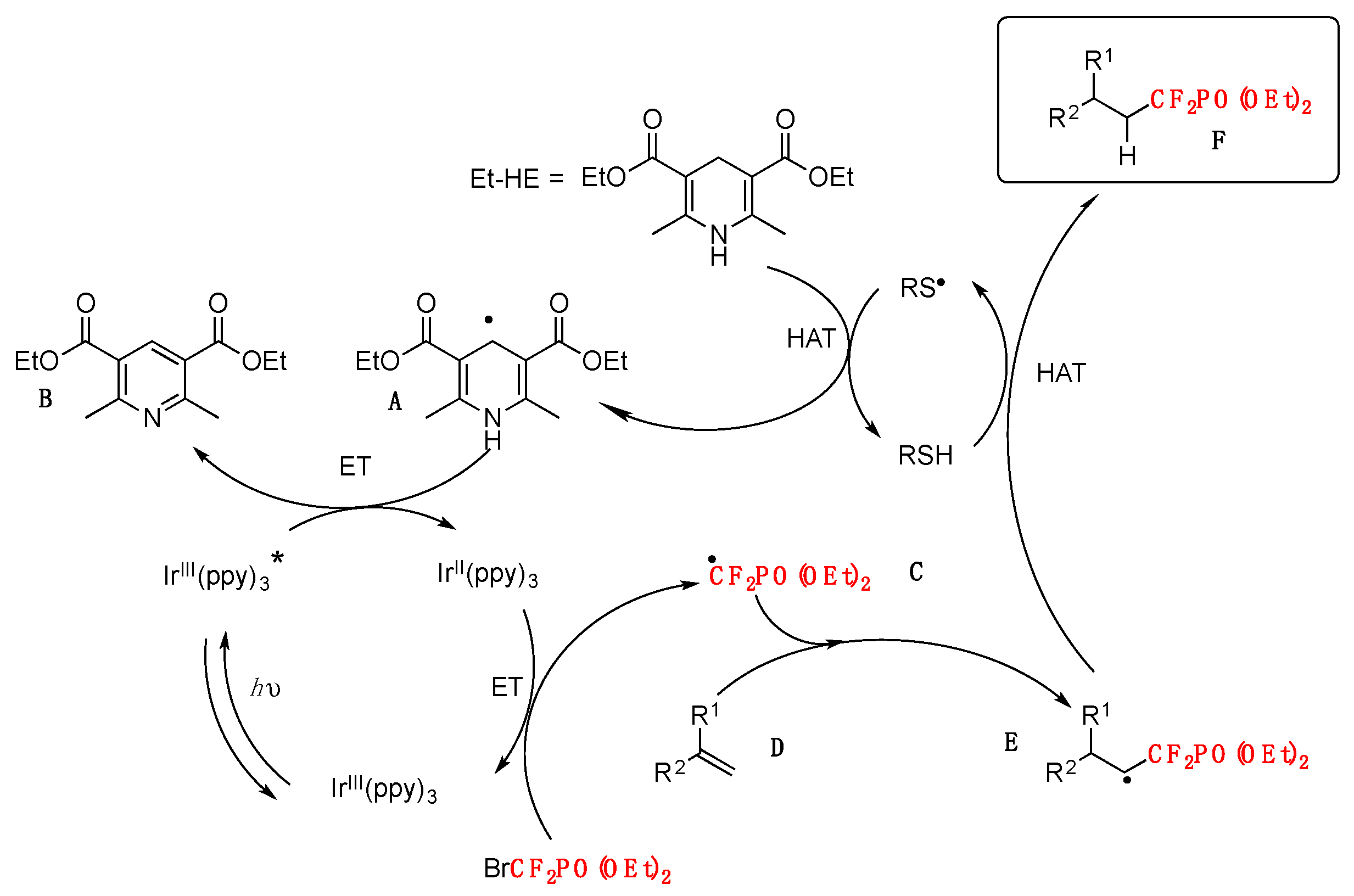
3.1. Difluoromethylation of Aliphatic Carbon–Carbon Multiple Bonds
3.2. Difluoromethylation of Carbon–Carbon Multiple Bonds and Ulterior Cyclization
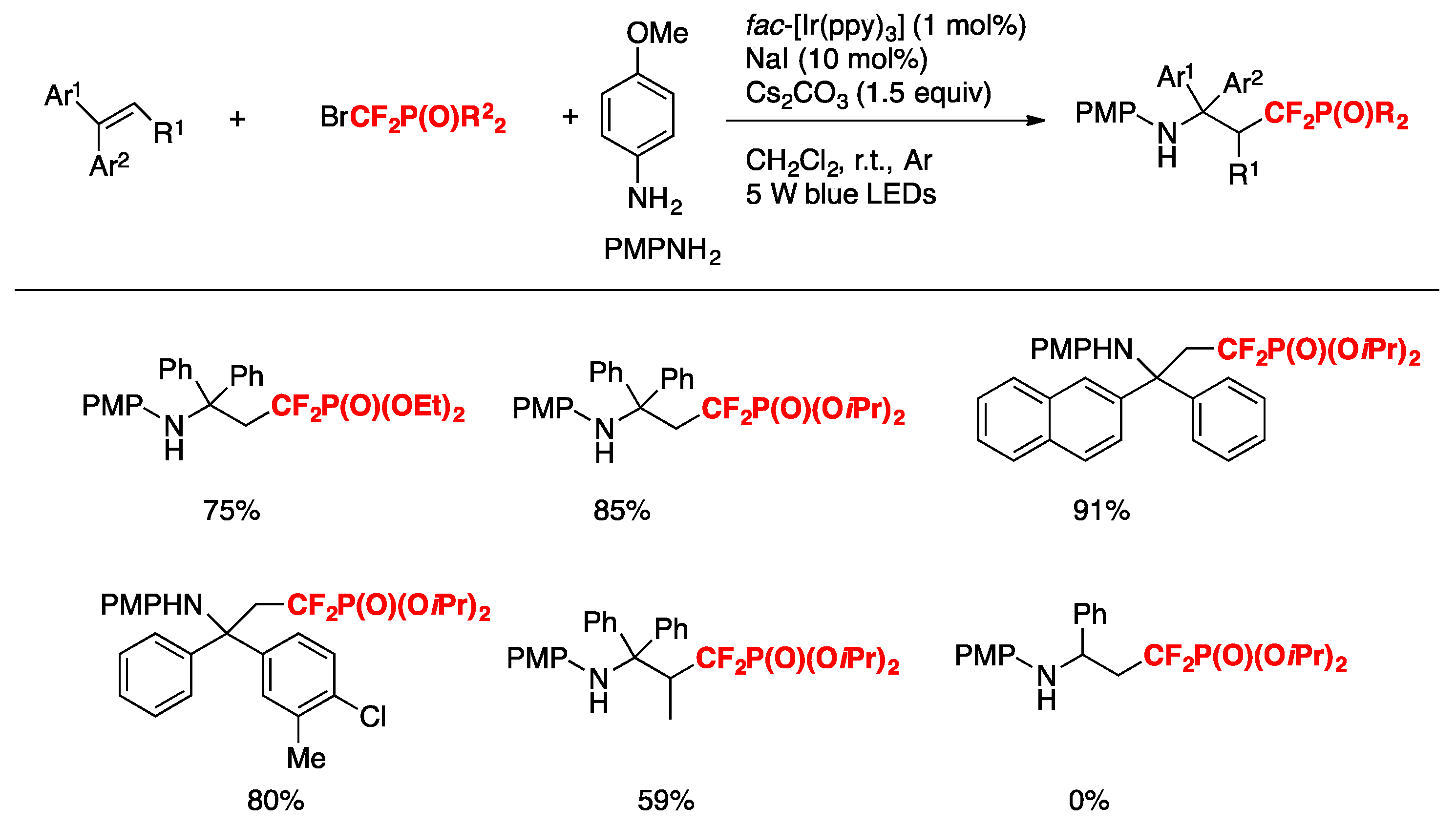
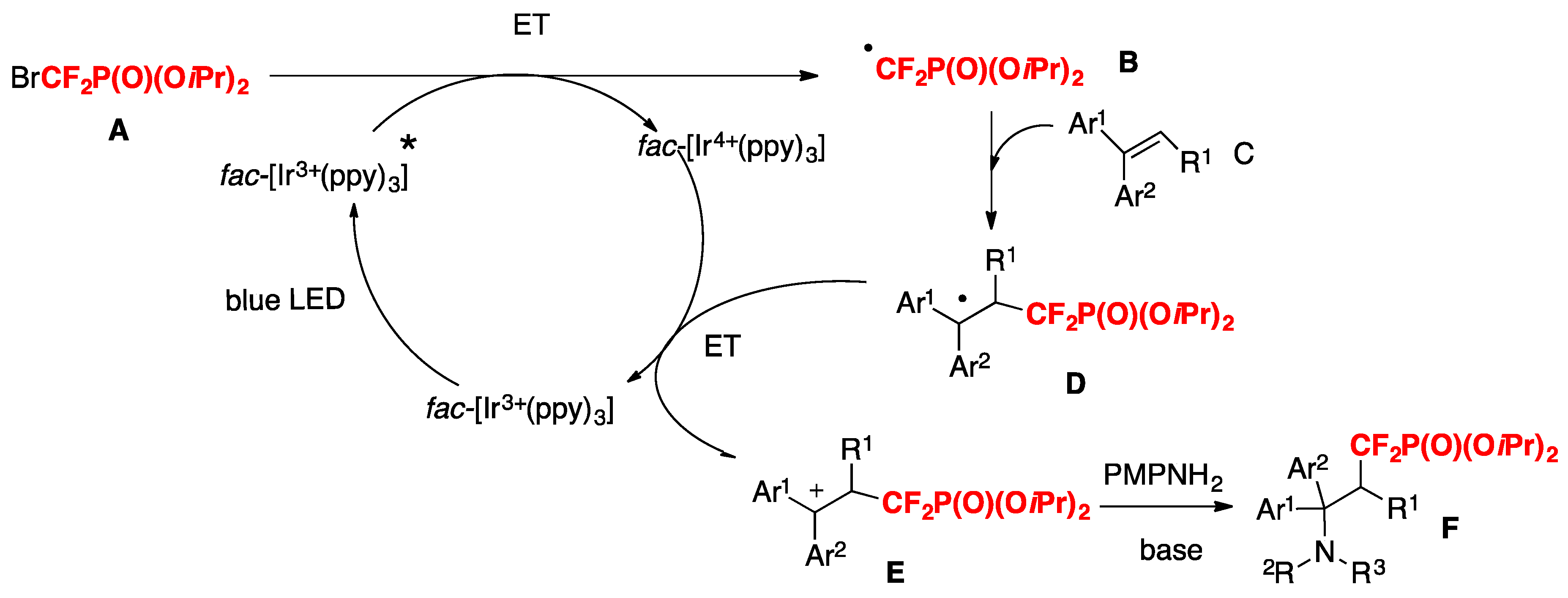
3.3. Difluoromethylation of Carbon–Carbon Multiple Bonds with Rearrangements
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Scheidt, F.; Neufeld, J.; Schäfer, M.; Thiehoff, C.; Gilmour, R. Catalytic Geminal Difluorination of Styrenes for the Construction of Fluorine-rich Bioisosteres. Org. Lett. 2018, 20, 8073–8076. [Google Scholar] [CrossRef] [PubMed]
- Drug Bank. Available online: https://http://www.drugbank.ca (accessed on 5 June 2017).
- Markovskij, L.N.; Pashinnik, V.E.; Kirsanov, A.V. Application of Dialkylaminosulfur Trifluorides in the Synthesis of Fluoroorganic Compounds. Synthesis 1973, 1973, 787–789. [Google Scholar] [CrossRef]
- Middleton, W.J. New fluorinating reagents. Dialkylaminosulfur fluorides. J. Org. Chem. 1975, 40, 574–578. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Ganesh, S.K.; Jones, J.P.; Kulkarni, A.; Masood, K.; Swabeck, J.K.; Olah, G.A. Copper-Mediated Difluoromethylation of (Hetero)aryl Iodides and β-Styryl Halides with Tributyl(difluoromethyl)stannane. Angew. Chem. Int. Ed. 2012, 51, 12090–12094. [Google Scholar] [CrossRef]
- Fier, P.S.; Hartwig, J.F. Copper-Mediated Difluoromethylation of Aryl and Vinyl Iodides. J. Am. Chem. Soc. 2012, 134, 5524–5527. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, Y.; Dixon, J.A.; Rodriguez, R.A.; Baxter, R.D.; Dixon, D.D.; Collins, M.R.; Blackmond, D.G.; Baran, P.S. A New Reagent for Direct Difluoromethylation. J. Am. Chem. Soc. 2012, 134, 1494–1497. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.-L.; Chen, Z.-H.; Xu, X.-H.; Qing, F.-L. Copper-mediated difluoromethylation of electron-poor aryl iodides at room temperature. Org. Chem. Front. 2014, 1, 774–776. [Google Scholar] [CrossRef]
- Matheis, C.; Jouvin, K.; Goossen, L. Sandmeyer Difluoromethylation of (Hetero-)Arenediazonium Salts. Org. Lett. 2014, 16, 5984–5987. [Google Scholar] [CrossRef]
- Feng, Z.; Min, Q.-Q.; Fu, X.-P.; An, L.; Zhang, X. Chlorodifluoromethane-triggered formation of difluoromethylated arenes catalysed by palladium. Nat. Chem. 2017, 9, 918–923. [Google Scholar] [CrossRef]
- Lu, C.; Lu, H.; Wu, J.; Shen, H.C.; Hu, T.; Gu, Y.; Shen, Q. Palladium-Catalyzed Difluoromethylation of Aryl Chlorides and Triflates and Its Applications in the Preparation of Difluoromethylated Derivatives of Drug/Agrochemical Molecules. J. Org. Chem. 2018, 83, 1077–1083. [Google Scholar] [CrossRef]
- Serizawa, H.; Ishii, K.; Aikawa, K.; Mikami, K. Copper-Catalyzed Difluoromethylation of Aryl Iodides with (Difluoromethyl)zinc Reagent. Org. Lett. 2016, 18, 3686–3689. [Google Scholar] [CrossRef] [PubMed]
- Levin, V.V.; Trifonov, A.L.; Zemtsov, A.A.; Struchkova, M.I.; Arkhipov, D.E.; Dilman, A.D. Difluoromethylene Phosphabetaine as an Equivalent of Difluoromethyl Carbanion. Org. Lett. 2014, 16, 6256–6259. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.K.S.; Krishnamoorthy, S.; Ganesh, S.K.; Kulkarni, A.; Haiges, R.; Olah, G.A. N-Difluoromethylation of Imidazoles and Benzimidazoles Using the Ruppert–Prakash Reagent under Neutral Conditions. Org. Lett. 2014, 16, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Thomoson, C.S.; Wang, L.; Dolbier, W.R. Use of fluoroform as a source of difluorocarbene in the synthesis of N-CF2H heterocycles and difluoromethoxypyridines. J. Fluorine Chem. 2014, 168, 34–39. [Google Scholar] [CrossRef]
- Mehta, V.P.; Greaney, M.F. S-, N-, and Se-Difluoromethylation Using Sodium Chlorodifluoroacetate. Org. Lett. 2013, 15, 5036–5039. [Google Scholar] [CrossRef] [PubMed]
- Heine, N.B.; Studer, A. Radical Difluoromethylation of Thiols with Difluoromethyl)triphenylphosphonium Bromide. Org. Lett. 2017, 19, 4150–4154. [Google Scholar] [CrossRef]
- Xu, W.; Abboud, K.A.; Ghiviriga, I.; Dolbier, W.R.; Rapp, M.; Wnuk, S.F. An unexpected reaction of trimethylsilyl fluorosulfonyldifluoroacetate (TFDA) with imidazoles. Formation of N-difluoromethylthioureas. Org. Lett. 2006, 8, 5549–5551. [Google Scholar] [CrossRef]
- Ando, M.; Wada, T.; Sato, N. Facile One-Pot Synthesis of N-Difluoromethyl-2-pyridone Derivatives. Org. Lett. 2006, 8, 3805–3808. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Dixon, J.A.; Rodriguez, R.A.; Baxter, R.D.; Dixon, D.D.; Collins, M.R.; Blackmond, D.G.; Baran, P.S. A New Reagent for Direct Difluoromethylation. J. Am. Chem. Soc. 2012, 134, 1494–1497. [Google Scholar] [CrossRef] [Green Version]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chemie Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, L.; Crisenza, G.E.M.; Melchiorre, P. Mechanistic Studies in Photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 3730–3747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAtee, R.C.; McClain, E.J.; Stephenson, C.R.J. Illuminating Photoredox Catalysis. Trends Chem. 2019, 1, 111–125. [Google Scholar] [CrossRef]
- Marchini, M.; Bergamini, G.; Cozzi, P.G.; Ceroni, P.; Balzani, V. Photoredox Catalysis: The Need to Elucidate the Photochemical Mechanism. Angew. Chem. Int. Ed. 2017, 56, 12820–12821. [Google Scholar] [CrossRef] [PubMed]
- Lowry, M.S.; Hudson, W.R.; Pascal, R.A.; Bernhard, S. Accelerated Luminophore Discovery through Combinatorial Synthesis. J. Am. Chem. Soc. 2004, 126, 14129–14135. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Teegardin, K.; Kelly, M.; Prasad, K.S.; Krishnan, S.; Weaver, J.D. Facile synthesis and complete characterization of homoleptic and heteroleptic cyclometalated Iridium(III) complexes for photocatalysis. J. Organomet. Chem. 2015, 776, 51–59. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6929. [Google Scholar] [CrossRef]
- Twilton, J.; Le, C.; Zhang, P.; Shaw, M.H.; Evans, R.W.; MacMillan, D.W.C. The merger of transition metal and photocatalysis. Nat. Chem. 2017. [Google Scholar] [CrossRef]
- Yerien, D.E.; Barata-Vallejo, S.; Postigo, A. Difluoromethylation Reactions of Organic Compounds. Chemistry 2017, 23, 14676–14701. [Google Scholar] [CrossRef]
- Rong, J.; Ni, C.; Hu, J. Metal-Catalyzed Direct Difluoromethylation Reactions. Asian J. Org. Chem. 2017, 6, 139–152. [Google Scholar] [CrossRef]
- Koike, T.; Akita, M. Recent progress in photochemical radical di- and monofluoromethylation. Org. Biomol. Chem. 2019, 17, 5413–5419. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Zhang, S.; Zhu, C.; Ruth, P.N.; Stalke, D.; Ackermann, L. Ruthenium(II)-Catalyzed meta C-H Mono- and Difluoromethylations by Phosphine/Carboxylate Cooperation. Angew. Chem. Int. Ed. 2017, 56, 2045–2049. [Google Scholar] [CrossRef] [PubMed]
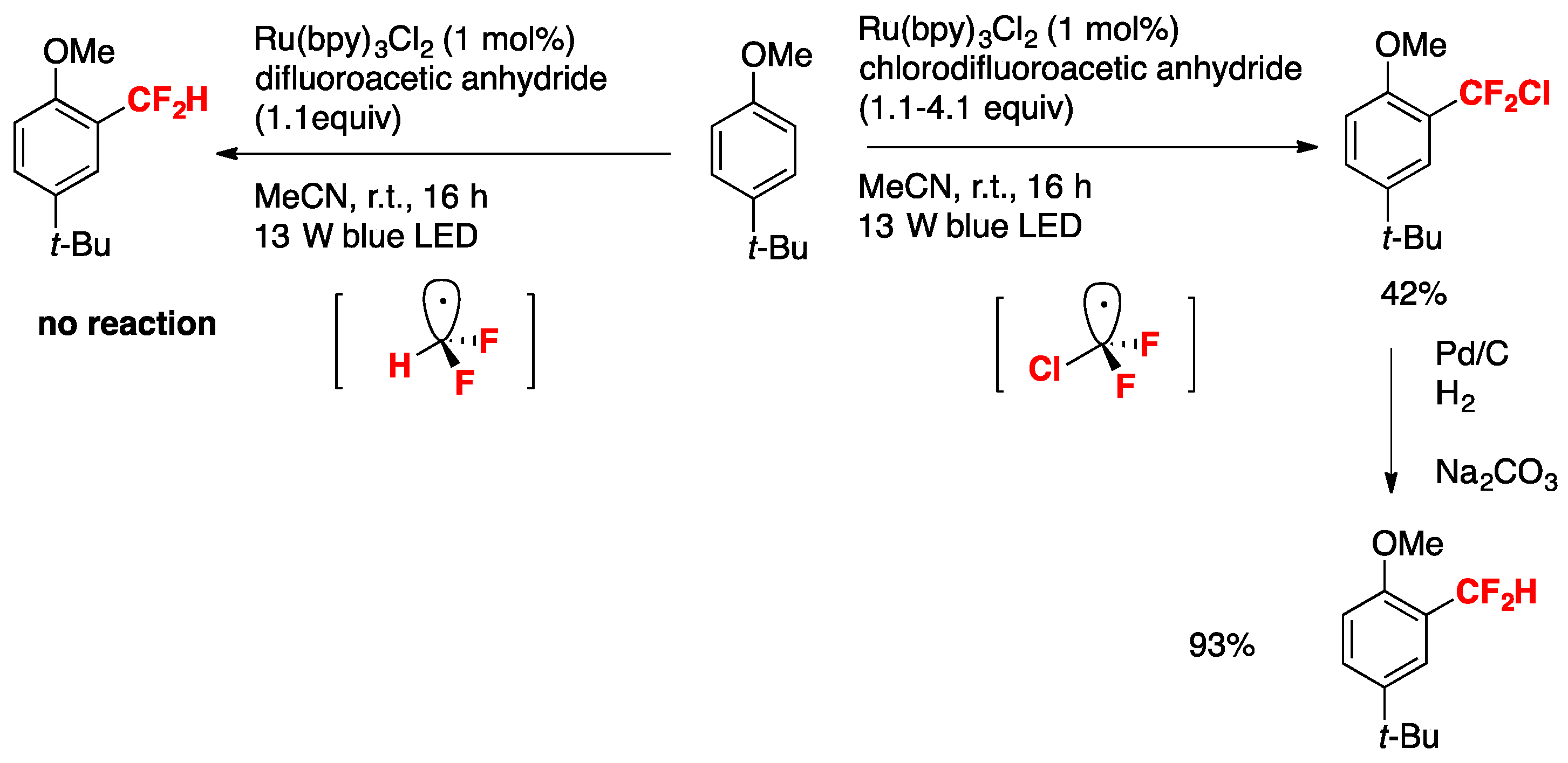
- McAtee, R.C.; Beatty, J.W.; McAtee, C.C.; Stephenson, C.R.J. Radical Chlorodifluoromethylation: Providing a Motif for (Hetero)arene Diversification. Org. Lett. 2018, 20, 3491–3495. [Google Scholar] [CrossRef] [PubMed]
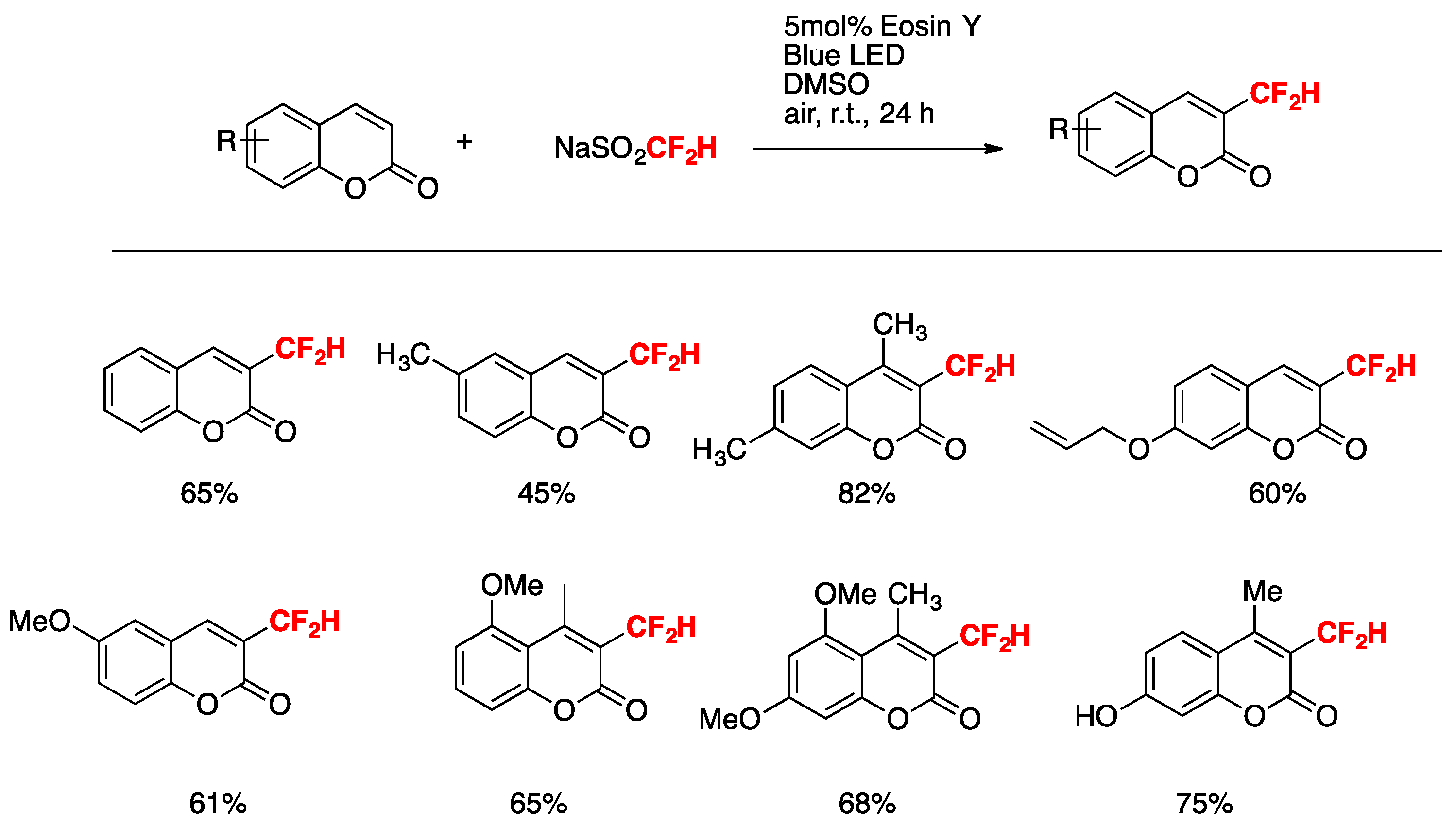
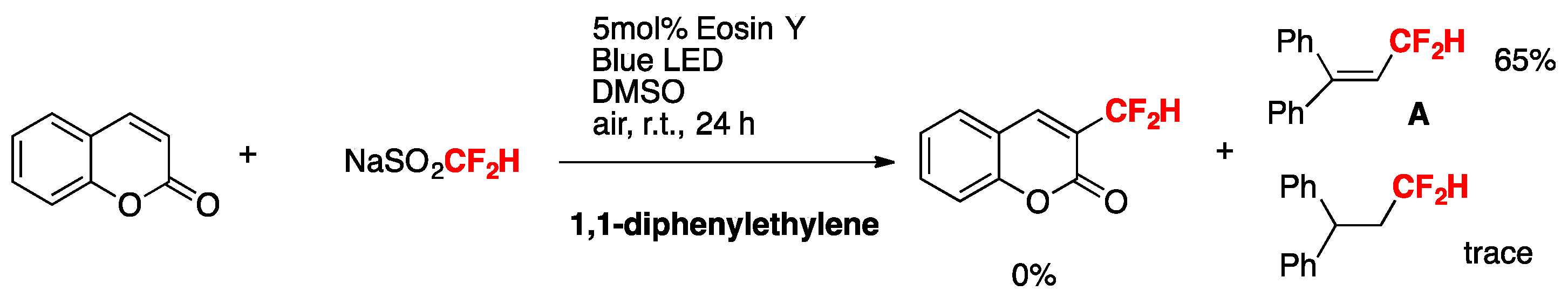
- Dai, P.; Yu, X.; Teng, P.; Zhang, W.-H.; Deng, C. Visible-Light- and Oxygen-Promoted Direct Csp2-H Radical Difluoromethylation of Coumarins and Antifungal Activities. Org. Lett. 2018, 20, 6901–6905. [Google Scholar] [CrossRef] [PubMed]
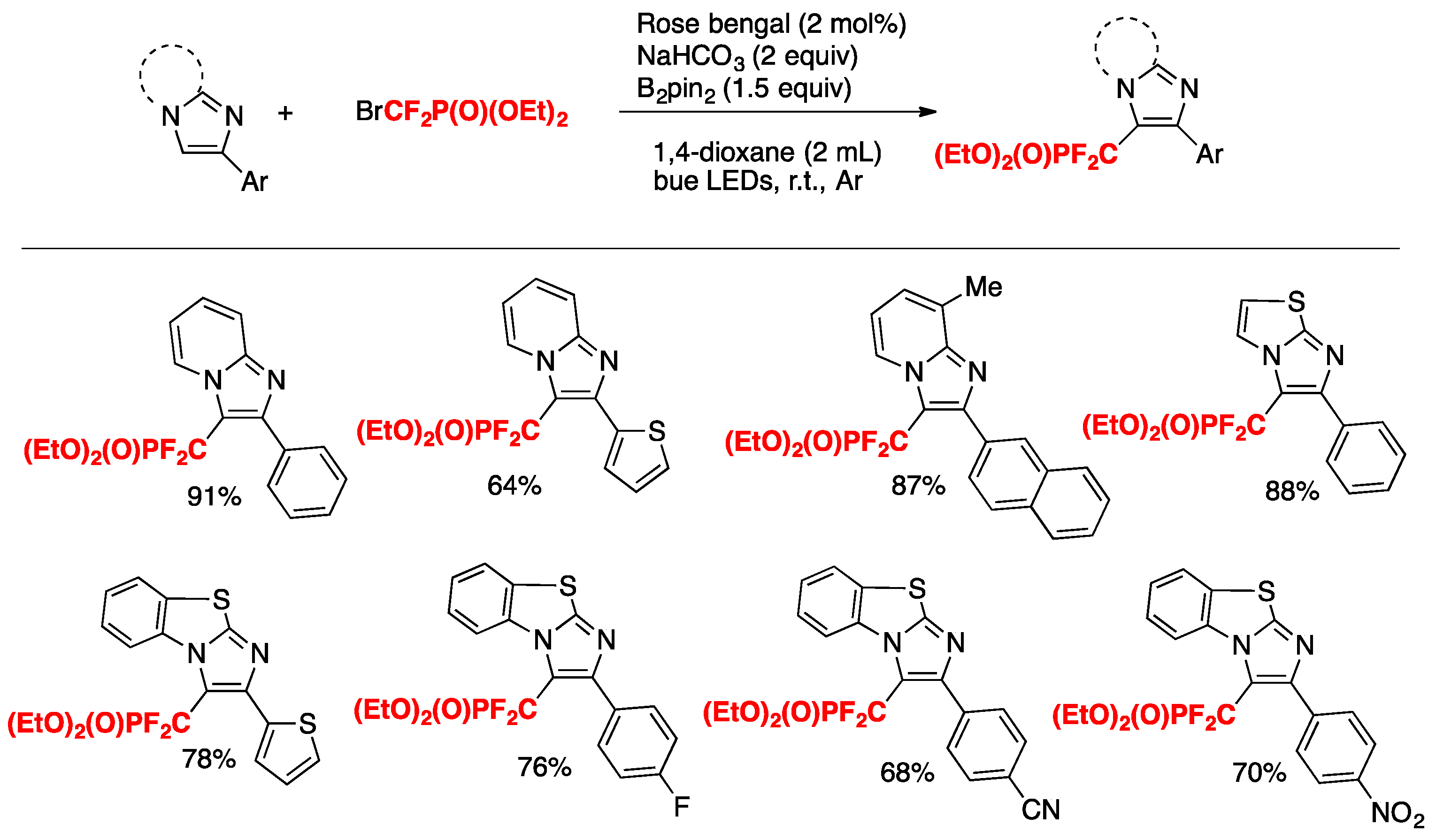
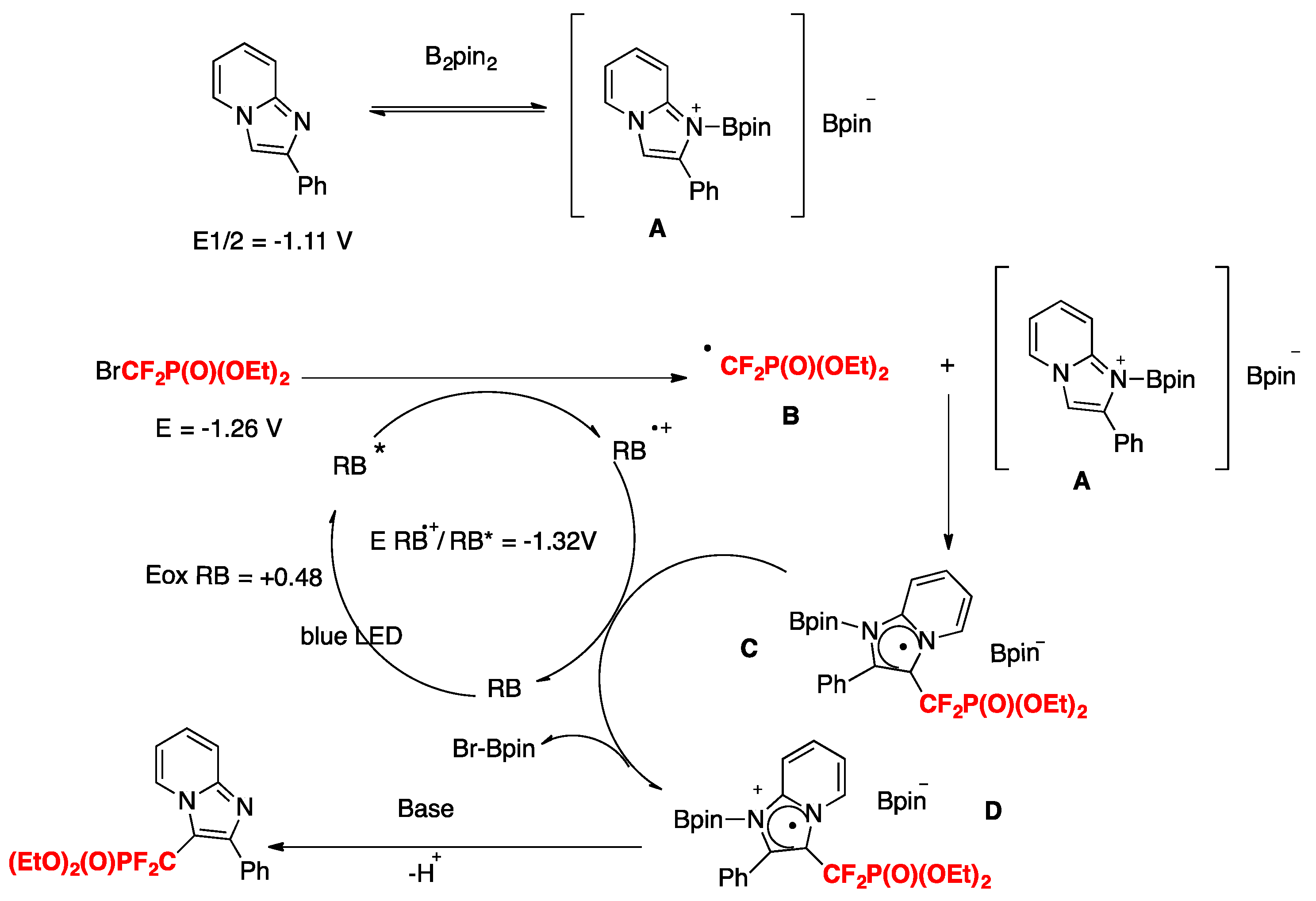
- Singsardar, M.; Mondal, S.; Laru, S.; Hajra, A. Organophotoredox-Catalyzed C(sp2)−H Difluoromethylenephosphonation of Imidazoheterocycles. Org. Lett. 2019, 21, 5606–5610. [Google Scholar] [CrossRef]
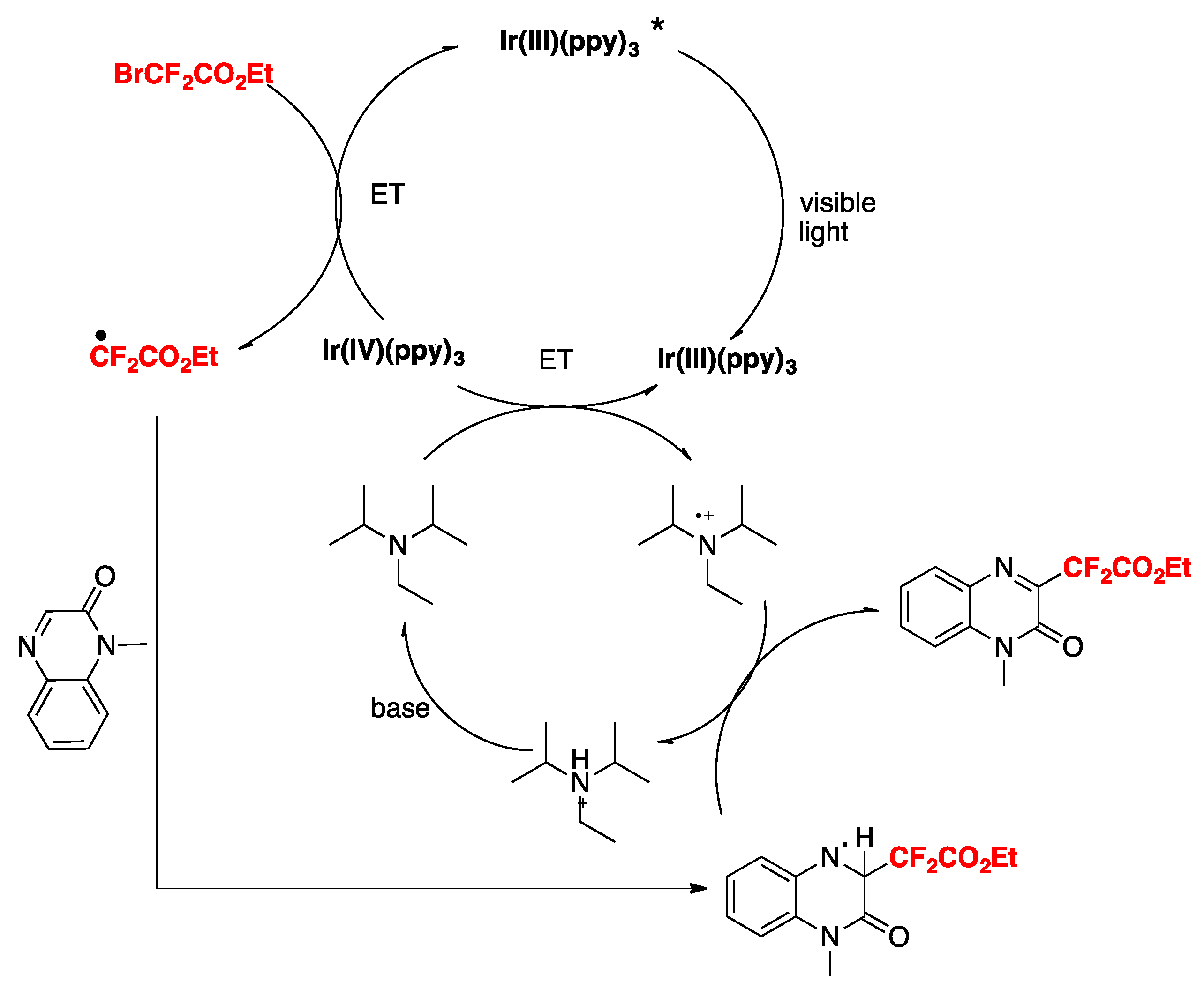
- Jin, C.; Zhuang, X.; Sun, B.; Li, D.; Zhu, R. Merging Visible-Light Photoredox and Organoamine Catalysis for the C-3 Difluoroalkylation of Quinoxalin-2(1H)-Ones. Asian J. Org. Chem. 2019, 8, 1490–1494. [Google Scholar] [CrossRef]
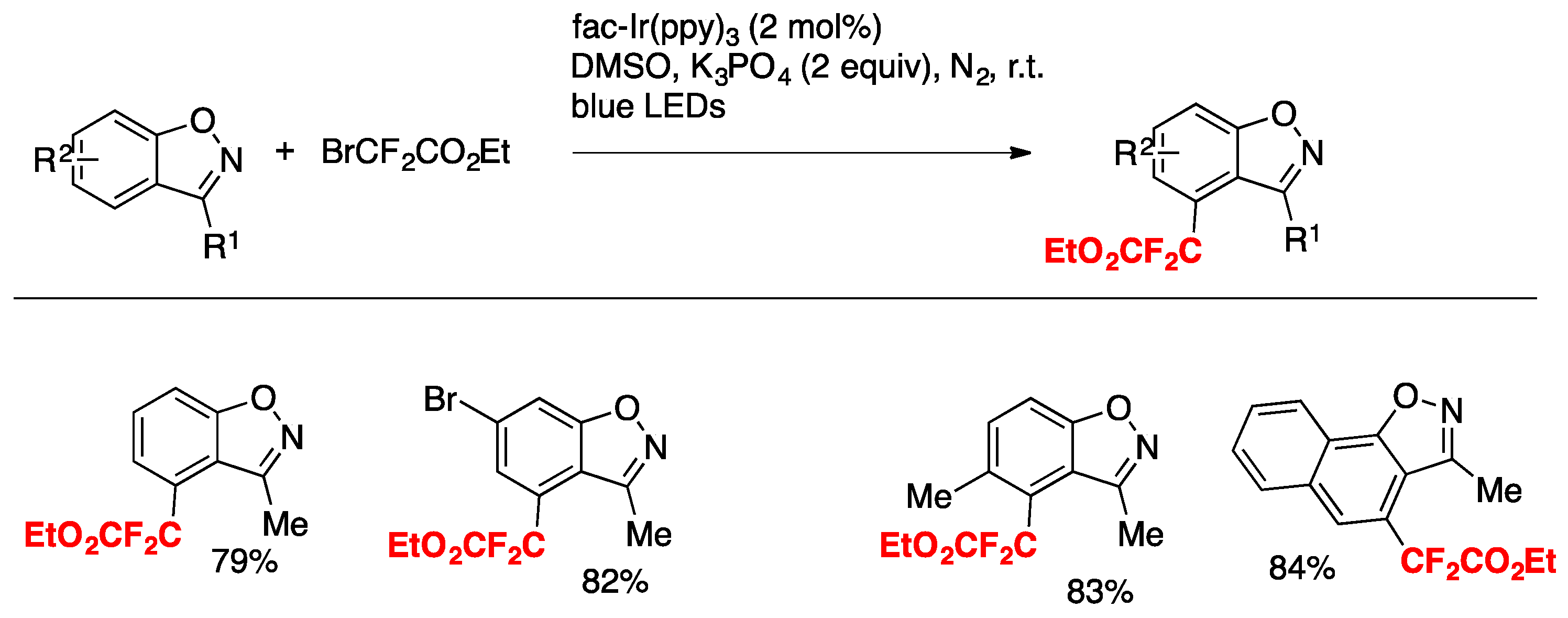
- Hua, M.-Q.; Moua, F.-Y.; Wang, N.-H.; Chen, Y.; Xiong, H.-Y.; Huang, Y.; Lei, C.-L.; Zhang, Q. Visible-light induced photocatalytic difluoromethylation of 3-substituted benzo[d]isoxazolesvia direct and regioselective C-H functionalization. Tet. Lett. 2018, 59, 4449–4453. [Google Scholar] [CrossRef]
- Rubinski, M.A.; Lopez, S.E.; Dolbier Jr, W.R. Direct access to 2-difluoromethylindoles via photoredox catalysis. J. Fluorine Chem. 2019, 224, 80–88. [Google Scholar] [CrossRef]
- Bacauanu, V.; Cardinal, S.; Yamauchi, M.; Kondo, M.; Fernández, D.F.; Remy, R.; MacMillan, D.W.C. Metallaphotoredox Difluoromethylation of Aryl Bromides. Angew. Chem. Int. Ed. 2018, 57, 12543–12548. [Google Scholar] [CrossRef]
- Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies; CRC: Boca Raton, FL, USA, 2007. [Google Scholar]
- Box, G.E.; Hunter, W.G.; Hunder, J.S. Statistics for Experimenters, An Introduction to Design, Data Analysis, and Model Building; John Wiley and Sons: New York, NY, USA, 1978; pp. 630–644. [Google Scholar]
- Zhang, P.; Le, C.C.; MacMillan, D.W.C. Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling. J. Am. Chem. Soc. 2016, 138, 8084–8087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.-K.; Ding, W.F.-X.; Zhang, Y.-H. The nucleophilic silyl radical. Dual partner correlation analysis of the relative rates of bromine atom abstraction reactions as measured by rigorous methodologies. Tetrahedron 1997, 53, 8479–8490. [Google Scholar] [CrossRef]
- Zuo, Z.; Ahneman, D.T.; Chu, L.; Terrett, J.A.; Doyle, A.G.; MacMillan, D.W.C. Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides. Science 2014, 345, 437–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halazy, S.; Ehrhard, A.; Danzin, C. 9-(Difluorophosphonoalkyl)guanines as a new class of multisubstrate analog inhibitors of purine nucleoside phosphorylase. J. Am. Chem. Soc. 1991, 113, 315–317. [Google Scholar] [CrossRef]
- Diab, S.A.; Sene, A.; Pfund, E.; Lequeux, T. Efficient Synthesis of Fluorophosphonylated Alkyles by Ring-Opening Reaction of Cyclic Sulfates. Org. Lett. 2008, 10, 3895–3898. [Google Scholar] [CrossRef] [PubMed]
- Na, Z.; Pan, S.; Uttamchandani, M.; Yao, S.Q. Discovery of cell-permeable inhibitors that target the BRCT domain of BRCA1 protein by using a small-molecule microarray. Angew. Chem. Int. Ed. 2014, 53, 8421–8426. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, J.; Wang, Y.; Wang, C.; Liu, X.; Xu, Z.; Lin, L.; Wang, R. Transition-Metal-Free Dehydrosilylative Difluoroamidation of Tetrahydroisoquinolines under Mild Conditions. Org. Lett. 2015, 17, 4212–4215. [Google Scholar] [CrossRef]
- Ye, Z.; Gettys, K.E.; Shen, X.; Dai, M. Copper-Catalyzed Cyclopropanol Ring Opening Csp3–Csp3 Cross-Couplings with (Fluoro)Alkyl Halides. Org. Lett. 2015, 17, 6074–6077. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-H.; Cao, Z.-Y.; Zhou, J. Nucleophilic Difluoromethylenation of Ketones Using Diethyl (Difluoro(trimethylsilyl)methyl)phosphonate Mediated by 18-Crown-6 Ether/KOAc. J. Org. Chem. 2016, 81, 7807–7816. [Google Scholar] [CrossRef]
- Cocaud, C.; Nicolas, C.; Poisson, T.; Pannecoucke, X.; Legault, C.Y.; Martin, O.R. Tunable Approach for the Stereoselective Synthesis of 1-C-Diethylphosphono(difluoromethyl) Iminosugars as Glycosyl Phosphate Mimics. J. Org. Chem. 2017, 82, 2753–2763. [Google Scholar] [CrossRef]
- Graton, J.; Compain, G.; Besseau, F.; Bogdan, E.; Watts, J.M.; Mtashobya, L.; Wang, Z.; Weymouth-Wilson, A.; Galland, N.; Le Questel, J.-Y.; et al. Influence of Alcohol β-Fluorination on Hydrogen-Bond Acidity of Conformationally Flexible Substrates. Chem. Eur. J. 2017, 23, 2811–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.-W.; Min, Q.-Q.; Yu, L.-C.; Zhang, X. Tandem Difluoroalkylation-Arylation of Enamides Catalyzed by Nickel. Angew. Chem. Int. Ed. 2016, 55, 12270–12274. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chen, F.; Zhang, X. Copper catalyzed cross-coupling of iodobenzoates with bromozinc-difluorophosphonate. Org. Lett. 2012, 14, 1938–1941. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Min, Q.-Q.; Xiao, Y.-L.; Zhang, B.; Zhang, X. Palladium-catalyzed difluoroalkylation of aryl boronic acids: A new method for the synthesis of aryldifluoromethylated phosphonates and carboxylic acid derivatives. Angew. Chem. Int. Ed. 2014, 53, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xiao, Y.-L.; Zhang, X. Palladium-catalyzed phosphonyldifluoromethylation of alkenes with bromodifluoromethylphosphonate. Org. Chem. Front 2016, 3, 466–469. [Google Scholar] [CrossRef] [Green Version]
- Yin, G.; Zhu, M.; Yang, G.; Wang, X.; Fu, W. Synthesis of difluoromethylenephosphonated oxindoles through visible-vight-induced radical cyclization of N-arylacrylamides. J. Fluorine Chem. 2016, 191, 63–69. [Google Scholar] [CrossRef]
- Wang, L.; Wei, X.-J.; Lei, W.-L.; Chen, H.; Wu, L.-Z.; Liu, Q. Direct C–H difluoromethylenephosphonation of arenes and heteroarenes with bromodifluoromethyl phosphonate via visible-light photocatalysis. Chem. Commun. 2014, 50, 15916–15919. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, M.V.; Bayle, A.; Besset, T.; Poisson, T.; Pannecoucke, X. Copper-Mediated Formation of Aryl, Heteroaryl, Vinyl and Alkynyl Difluoromethylphosphonates: A General Approach to Fluorinated Phosphate Mimics. Angew. Chem. Int. Ed. 2015, 54, 13406–13410. [Google Scholar] [CrossRef]
- Zhu, M.; Fu, W.; Zou, G.; Xu, C.; Wang, Z. Visible-light-mediated radical difluoromethylenephosphonation of 2-isocyanobiaryls with bromodifluoromethylphosphonate for the synthesis of 6-difluoromethylenephosphonyl-phenanthridines. J. Fluorine Chem. 2015, 180, 1–6. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, T.; Chen, F.; Mehrkens, N.; Rominger, F.; Rudolph, M.; Hashmi, A.S.K. Gold-Catalyzed Highly Selective Photoredox C(sp2)−H Difluoroalkylation and Perfluoroalkylation of Hydrazones. Angew. Chem. Int. Ed. 2016, 55, 2934–2938. [Google Scholar] [CrossRef]
- Bo, Y.; Min, Z.; Xiang, F.; Xueyang, Y.; Fanhong, W. A convenient synthesis of 4-fluoroalkyl-2-oxazolidinone. Chin. J. Org. Chem. 2013, 33, 1088. [Google Scholar]
- Kondratov, I.S.; Bugera, M.Y.; Tolmachova, N.A.; Posternak, G.G.; Daniliuc, C.G.; Haufe, G. Radical Reactions of Alkyl 2-Bromo-2,2-difluoroacetates with Vinyl Ethers: “Omitted” Examples and Application for the Synthesis of 3,3-Difluoro-GABA. J. Org. Chem. 2015, 80, 12258–12264. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, I.S.; Bugera, M.Y.; Tolmachova, N.A.; Posternak, G.G.; Daniliuc, C.G.; Haufe, G. Stereoselective synthesis of α,α-difluoro-β,γ-alkenyl ketones by free-radical reaction of iododifluoromethyl ketones with alkynes. Tetrahedron 2017, 73, 3478–3484. [Google Scholar]
- Lequeux, T.; Lebouc, F.; Lopin, C.; Yang, H.; Gouhier, G.; Piettre, S.R. Sulfanyl- and Selanyldifluoromethylphosphonates as a Source of Phosphonodifluoromethyl Radicals and Their Addition onto Alkenes. Org. Lett. 2001, 3, 185–188. [Google Scholar] [CrossRef]
- Pignard, S.; Lopin, C.; Gouhier, G.; Piettre, S.R. Sulfanyl- and Selanyldifluoromethylphosphonates as a Source of Phosphonodifluoromethyl Radicals and Their Addition onto Alkenes. J. Org. Chem. 2006, 71, 31–37. [Google Scholar] [CrossRef]
- Nagura, H.; Murakami, S.; Fuchigami, T. Photochemical generation of difluoromethyl radicals having various functional groups and their highly regioselective addition to olefins and aromatic substitution. Tetrahedron 2007, 63, 10237–10245. [Google Scholar] [CrossRef]
- Huang, W.; Chen, J.; Hong, D.; Chen, W.; Cheng, X.; Tian, Y.; Li, G. Hydrophosphonodifluoromethylation of Alkenes via Thiyl-Radical/Photoredox Catalysis. J. Org. Chem. 2018, 83, 578–587. [Google Scholar] [CrossRef]
- Yang, Q.; Li, C.; Qi, Z.-C.; Qiang, X.-Y.; Yang, S.-D. Photocatalyzed Intermolecular Aminodifluoromethylphosphonation of Alkenes: Facile Synthesis of α,α-Difluoro-γ-aminophosphonates. Chem. Eur. J. 2018, 24, 14363–14367. [Google Scholar] [CrossRef]
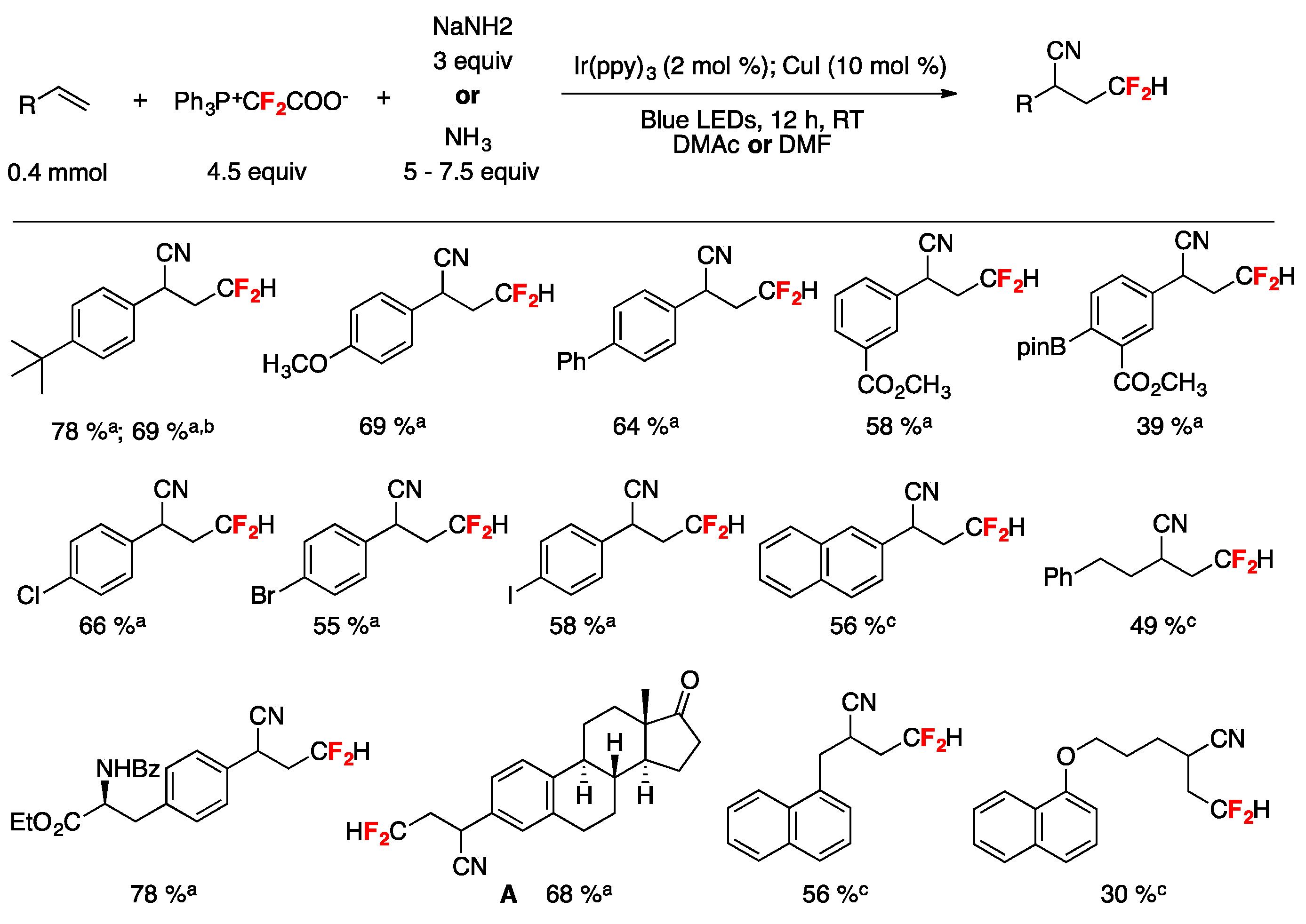
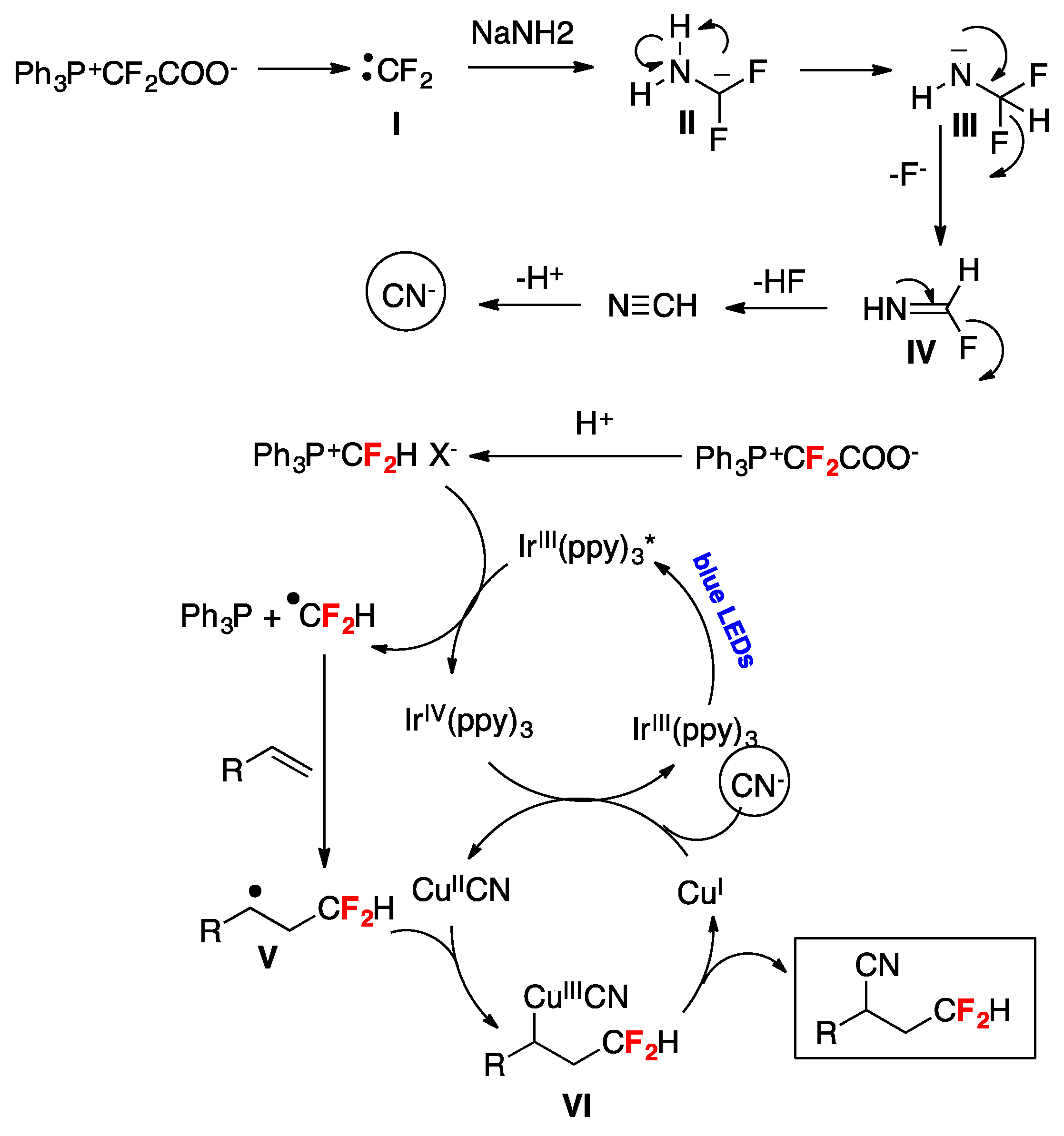
- Zhang, M.; Lin, J.H.; Xiao, J.C. Photocatalyzed Cyanodifluoromethylation of Alkenes. Angew. Chem. Int. Ed. 2019, 58, 6079–6083. [Google Scholar] [CrossRef]
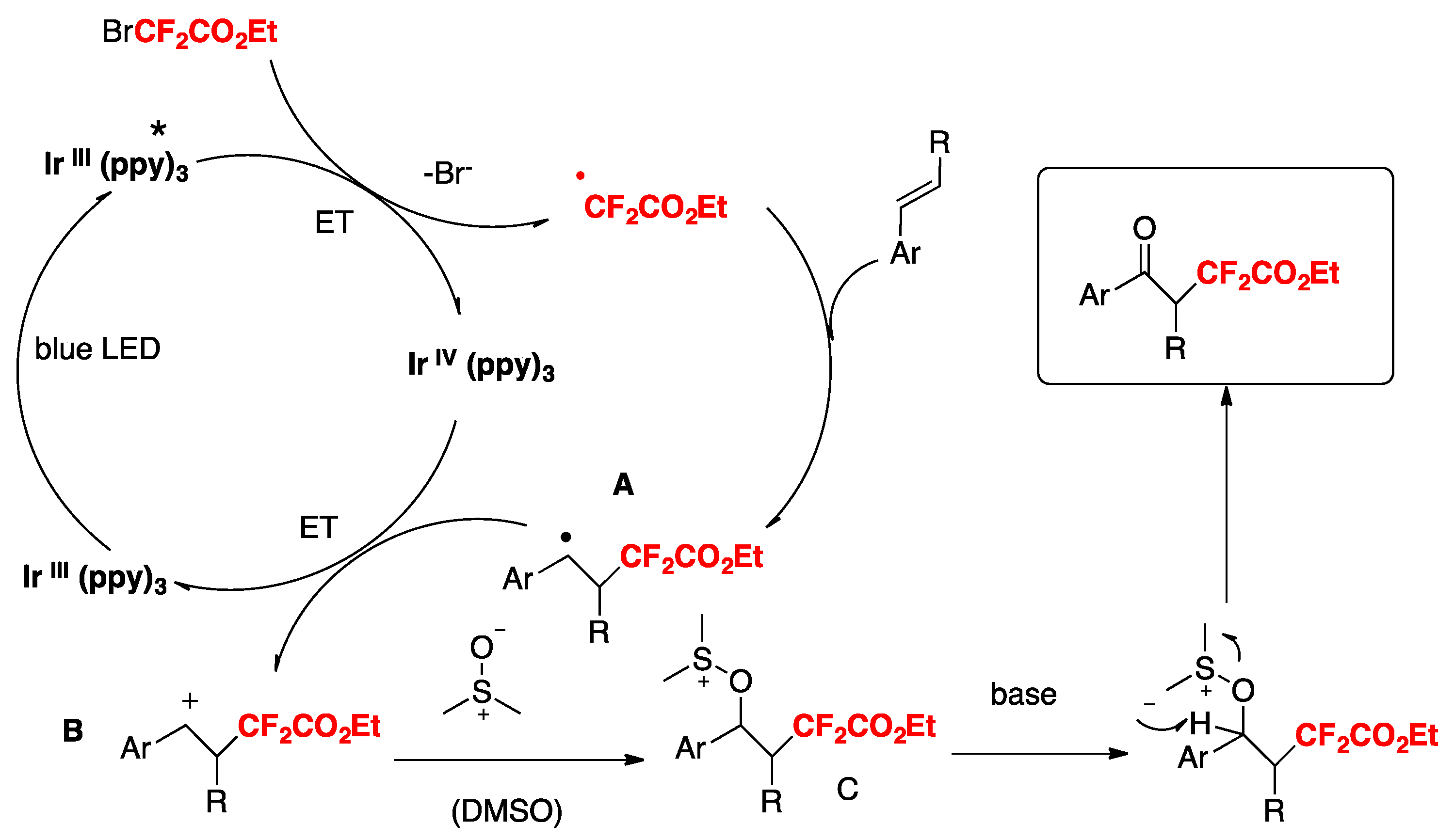
- Xia, Z.-H.; Gao, Z.-H.; Dai, L.; Ye, S. Visible-Light-Promoted Oxo-difluoroalkylation of Alkenes with DMSO as oxidant. J. Org. Chem. 2019, 84, 7388–7394. [Google Scholar] [CrossRef]
- Sambiago, C.; Noel, T. Flow Photochemistry: Shine some light on those tubes! Trends Chem. 2019. [Google Scholar] [CrossRef] [Green Version]
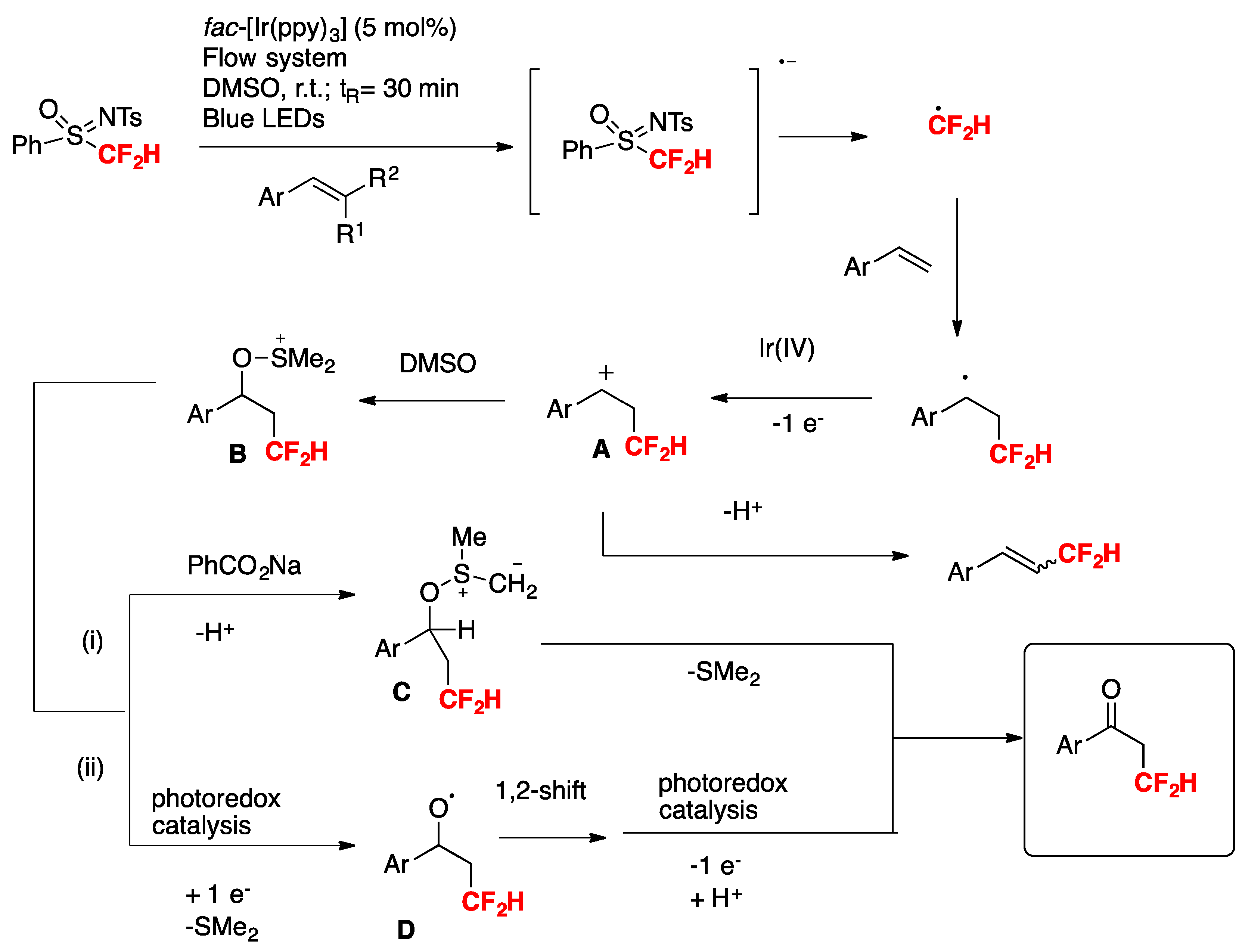
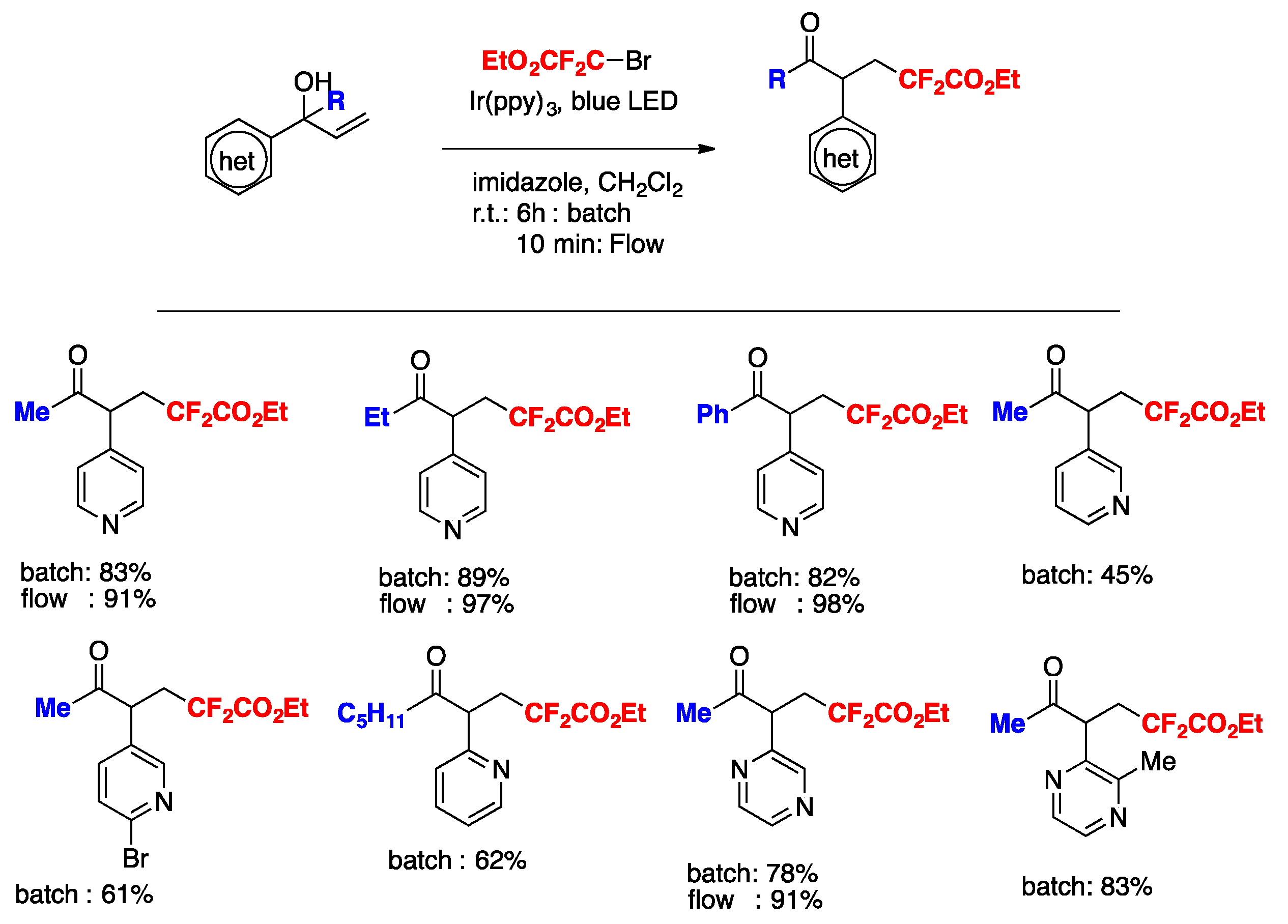
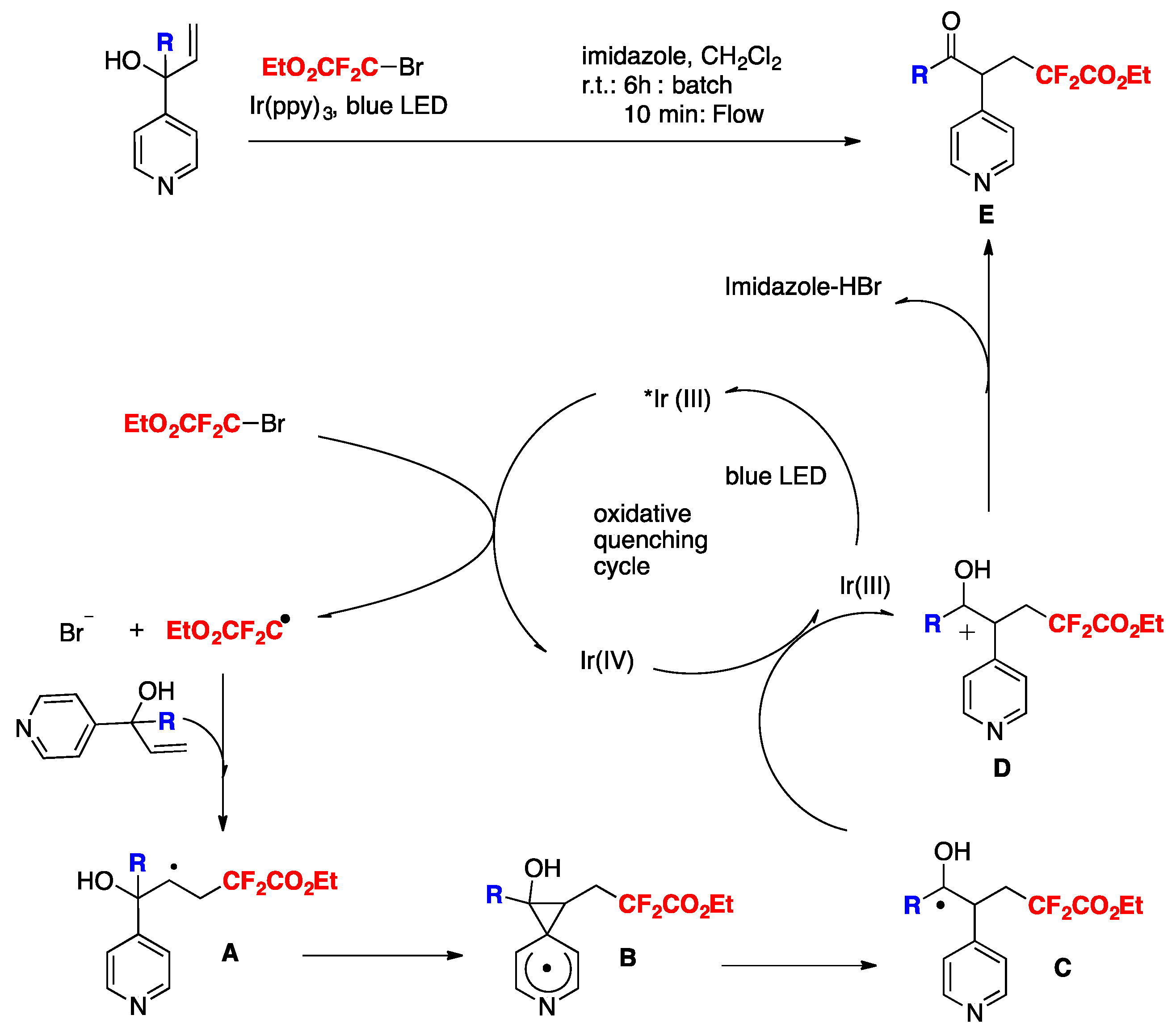
- Nakayama, Y.; Ando, G.; Abe, M.; Koike, T.; Akita, M. Keto-Difluoromethylation of Aromatic Alkenes by PhotoredoxCatalysis: Step-Economical Synthesis of α-CF2H-Substituted Ketones in Flow. ACS Catal. 2019, 9, 6555–6563. [Google Scholar] [CrossRef]
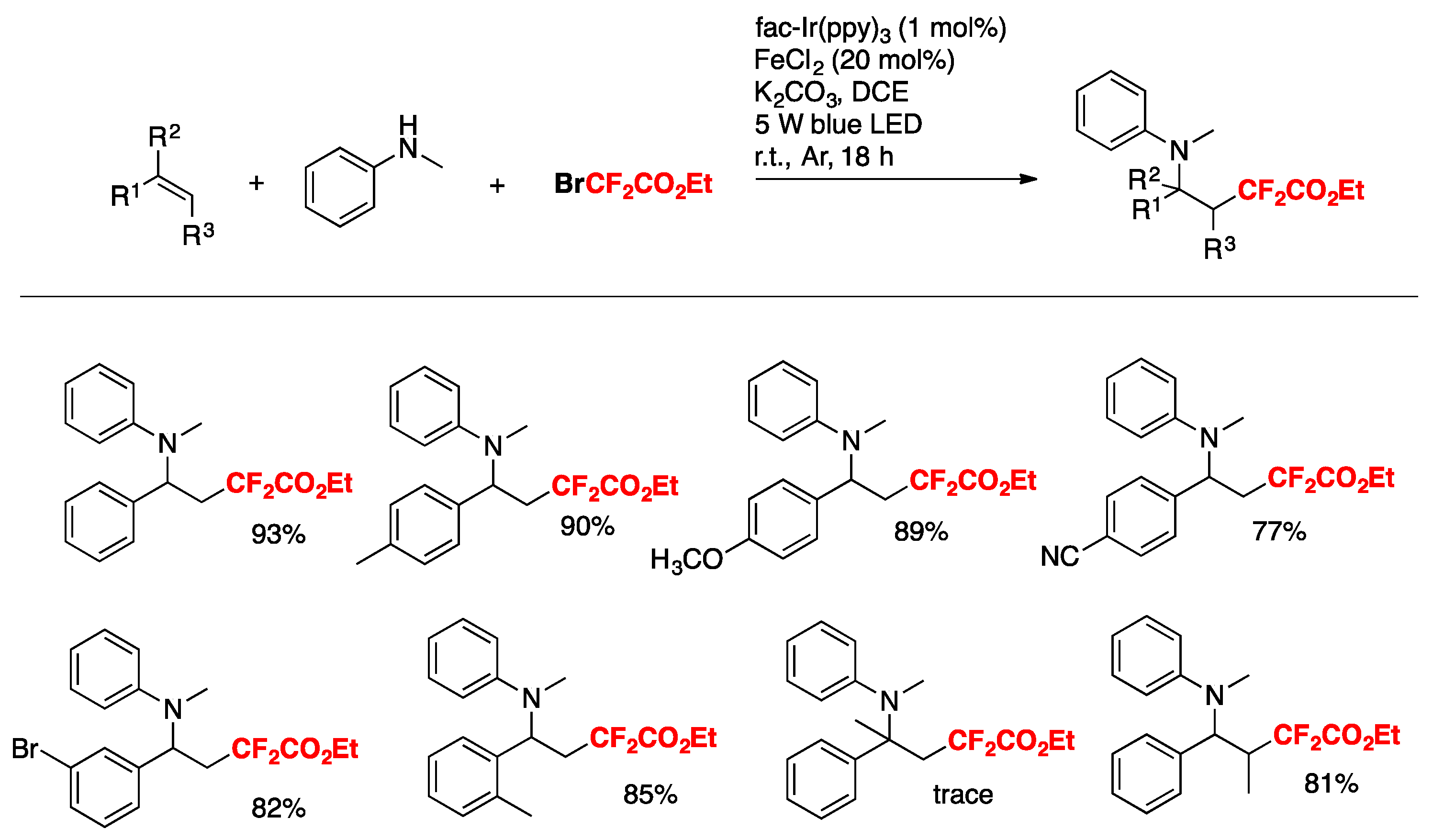
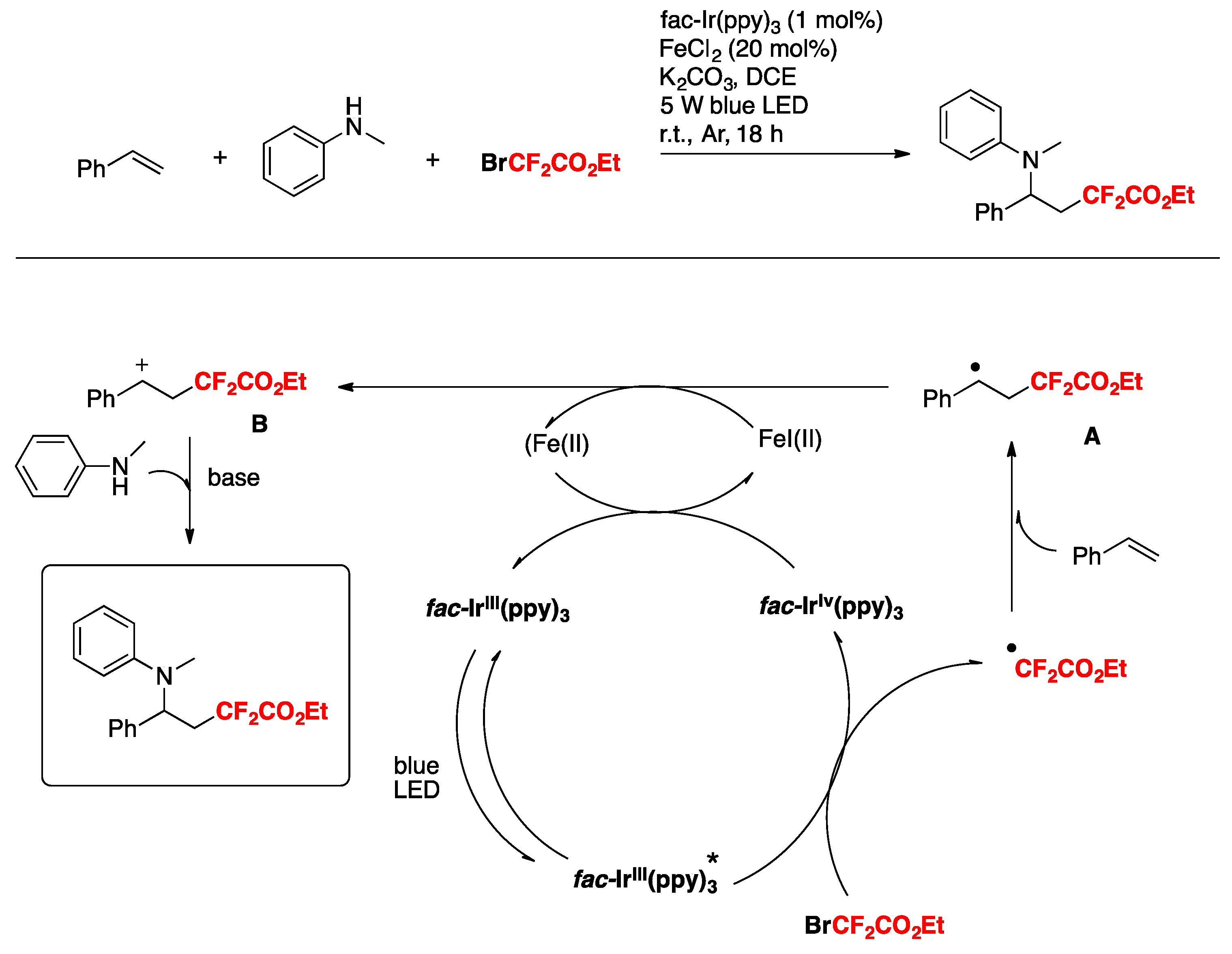
- Xu, R.; Cai, C. Three-Component Difluoroalkylamination of Alkenes Mediated by Photoredox and Iron Cooperative Catalysis. Org. Biomol. Chem. 2019, 17, 8541–8545. [Google Scholar] [CrossRef] [PubMed]
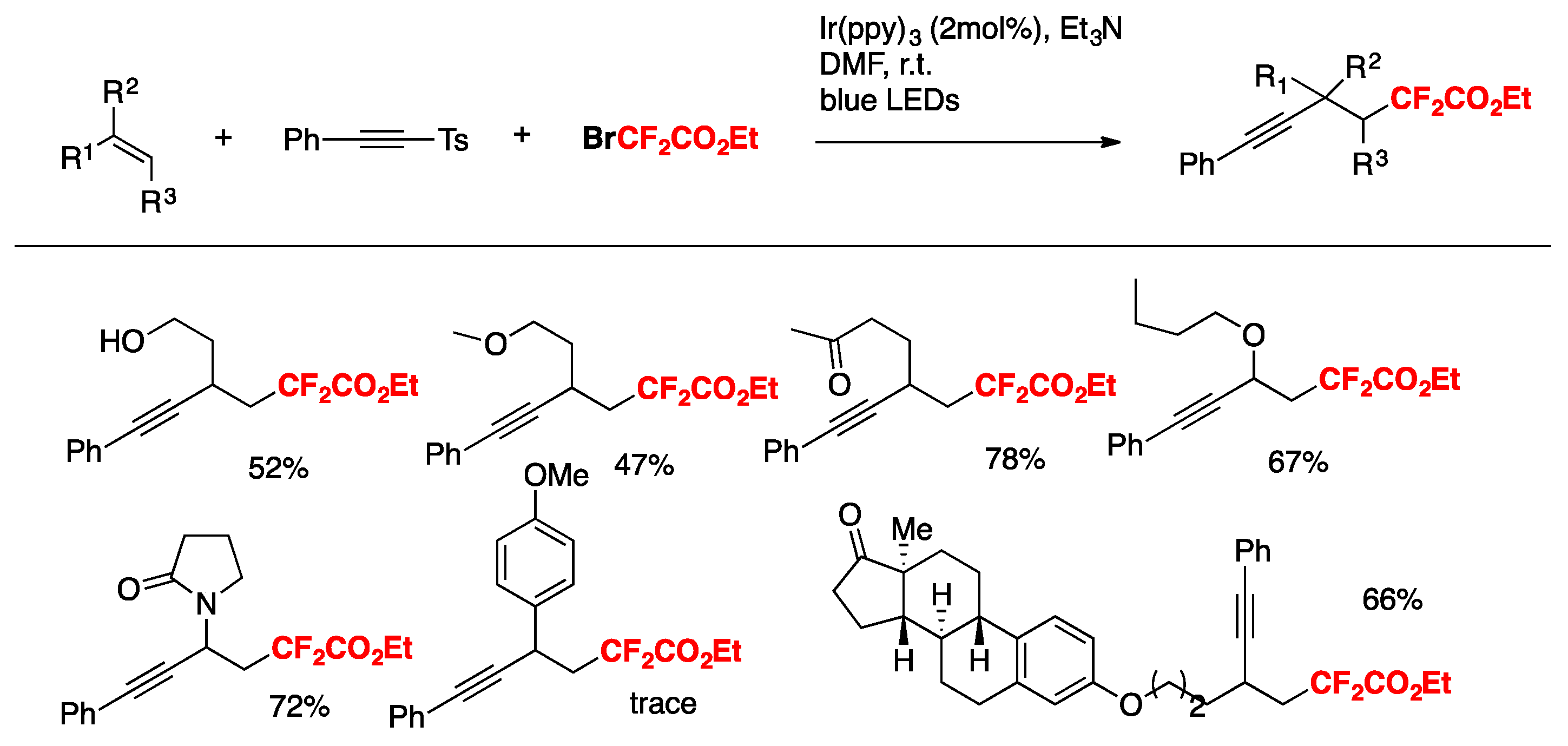
- Jin, W.; Wu, M.; Xiong, Z.; Zhu, G. Visible-light induced three-component alkynyl-difluoroalkylation of unactivated alkenes. Chem. Commun. 2018, 54, 7924–7927. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Tan, L.; Xu, Y.; Liu, P.; Sun, P. Cyanomethylation and Cyclization of Aryl Alkynoates with Acetonitrile under Transition-Metal-Free Conditions: Synthesis of 3-Cyanomethylated Coumarins. J. Org. Chem. 2018, 83, 6151–6161. [Google Scholar] [CrossRef] [PubMed]
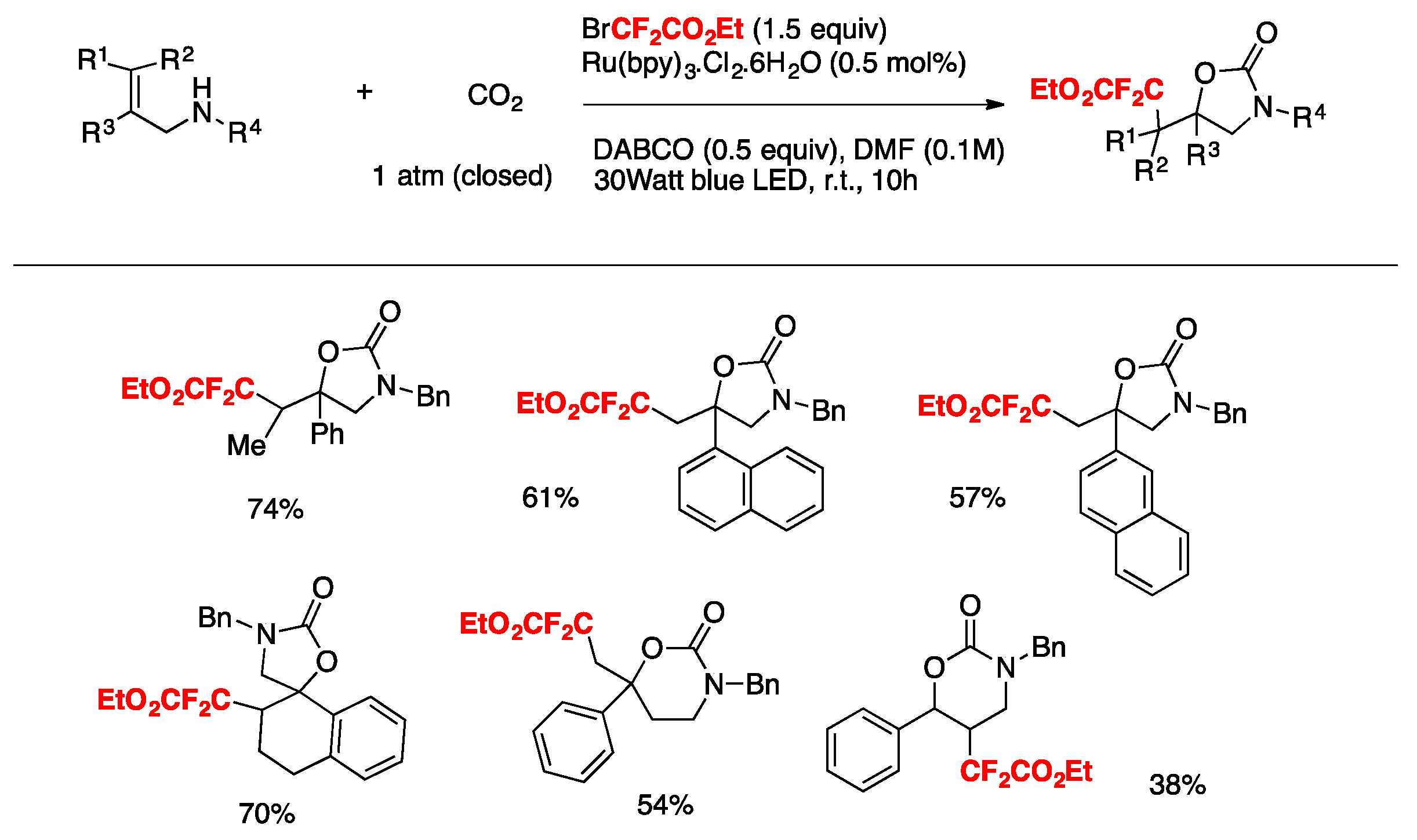
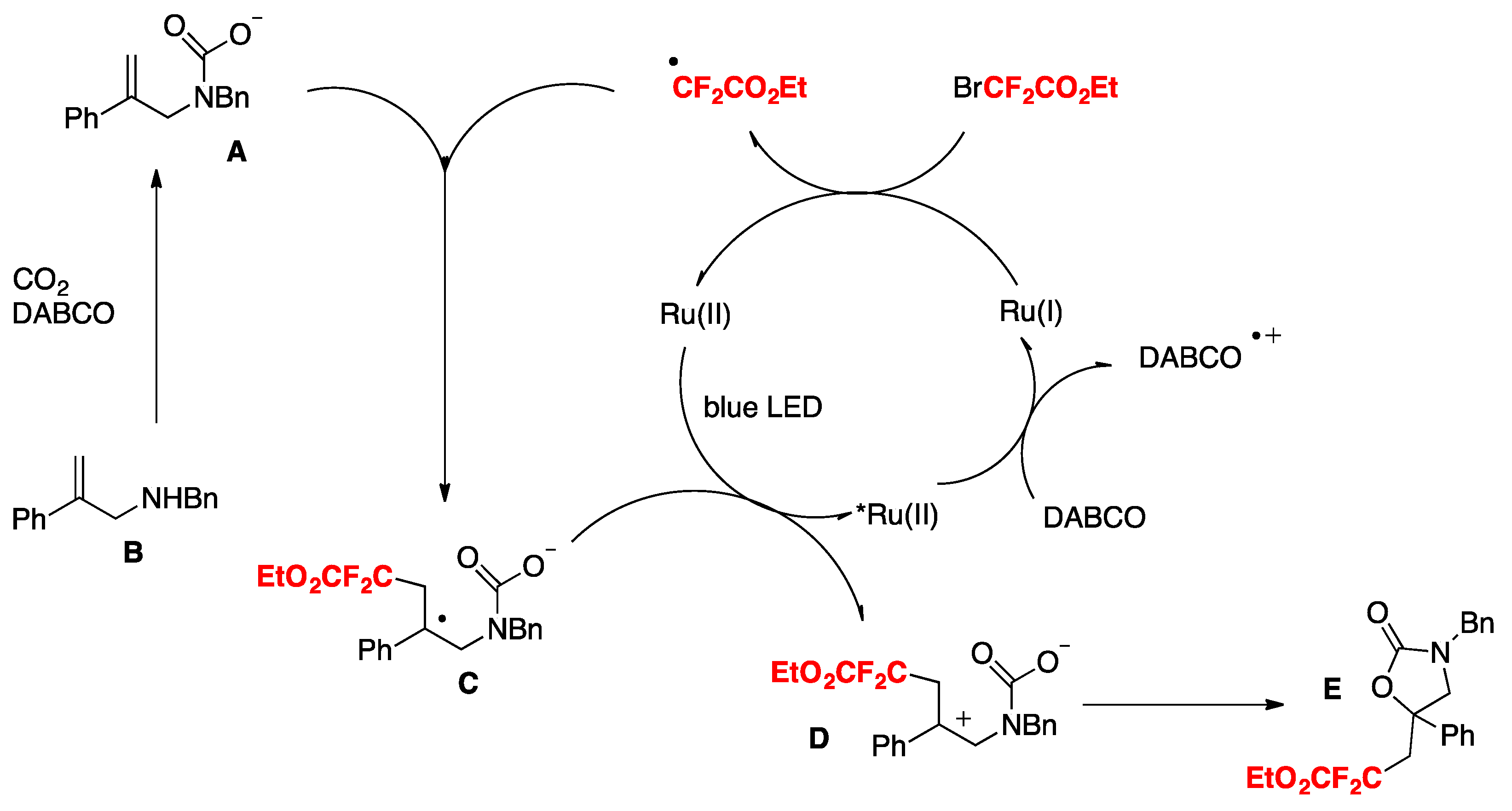
- Yin, Z.-B.; Ye, J.-H.; Zhou, W.-J.; Zhang, Y.-H.; Ding, L.; Gui, Y.-Y.; Yan, S.-S.; Li, J.; Yu, D.-G. Oxy-Difluoroalkylation of Allylamines with CO2 via Visible-Light Photoredox Catalysis. Org. Lett. 2018, 20, 190–193. [Google Scholar] [CrossRef] [PubMed]
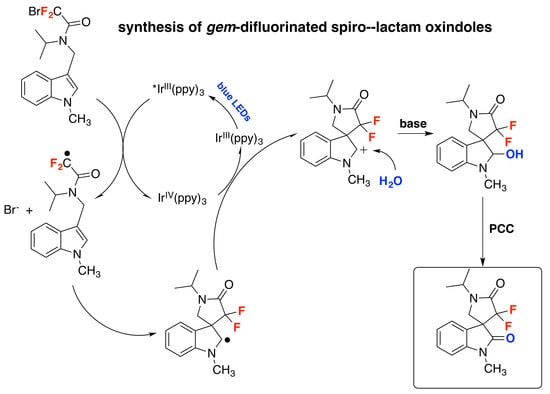
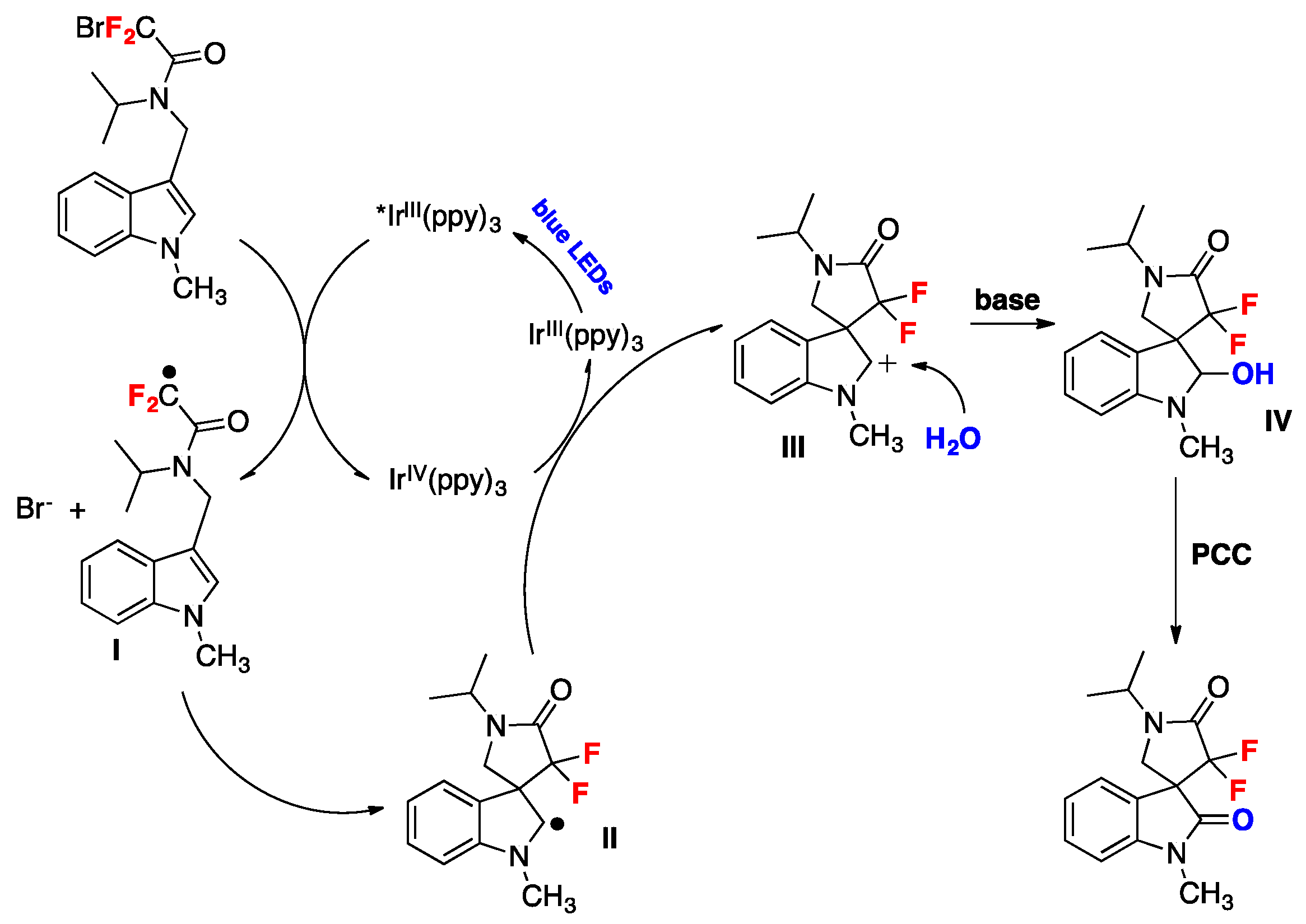
- Wang, Q.; Qu, Y.; Xia, Q.; Song, H.; Song, H.; Liu, Y.; Wang, Q. Synthesis of gem-Difluorinated Spiro-γ-lactam Oxindoles by Visible-Light-Induced Consecutive Difluoromethylative Dearomatization, Hydroxylation, and Oxidation. Chem. Eur. J. 2018, 24, 11283–11287. [Google Scholar] [CrossRef] [PubMed]
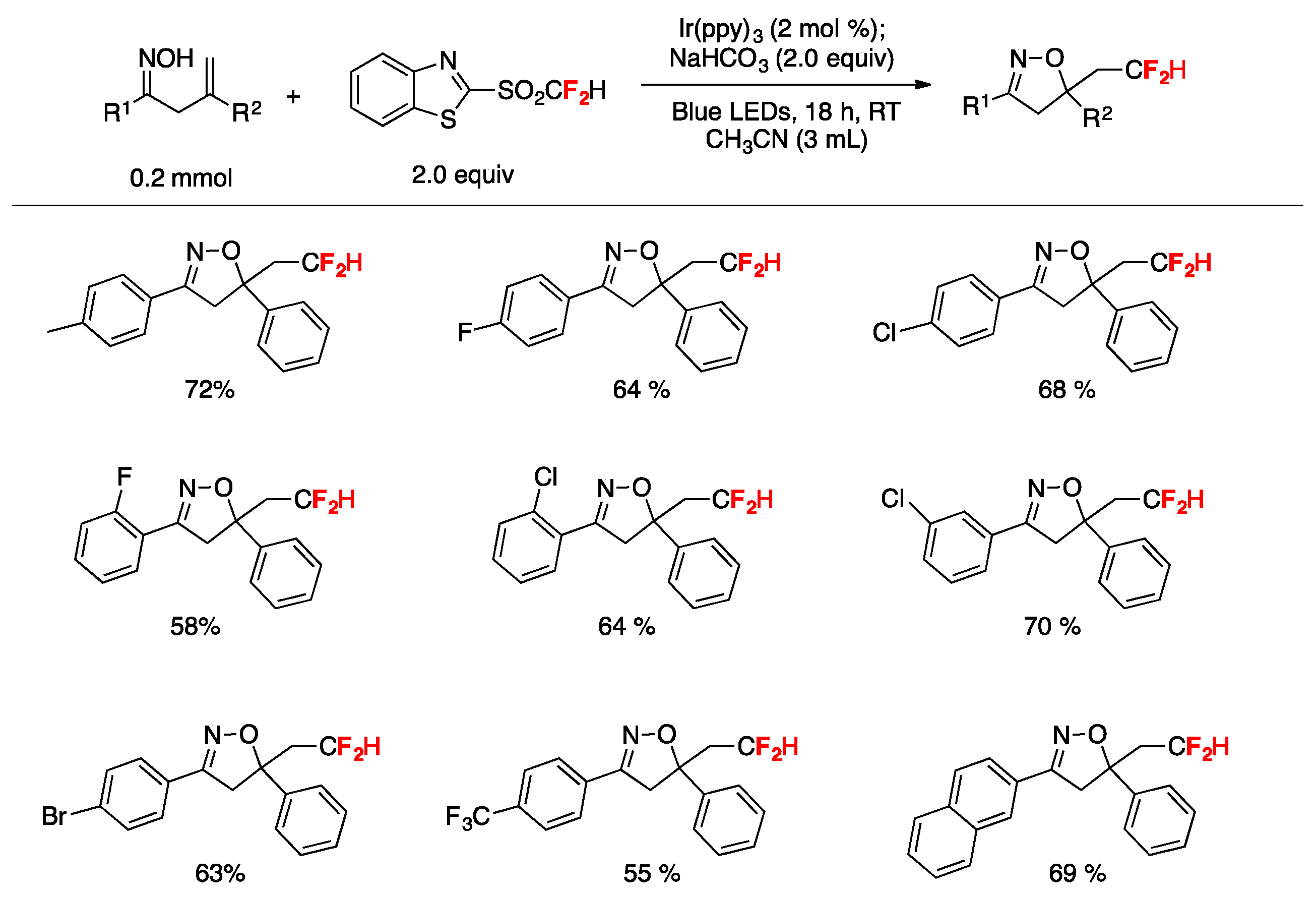
- Zhu, M.; Fun, W.; Guo, W.; Tian, Y.; Wang, Z.; Xu, C.; Ji, B. Visible-Light-Induced Radical Di- and Trifluoromethylation of β, γ-Unsaturated Oximes: Synthesis of Di- and Trifluoromethylated Isoxazolines. Eur. J. Org. Chem. 2019, 2019, 1614–1619. [Google Scholar] [CrossRef]
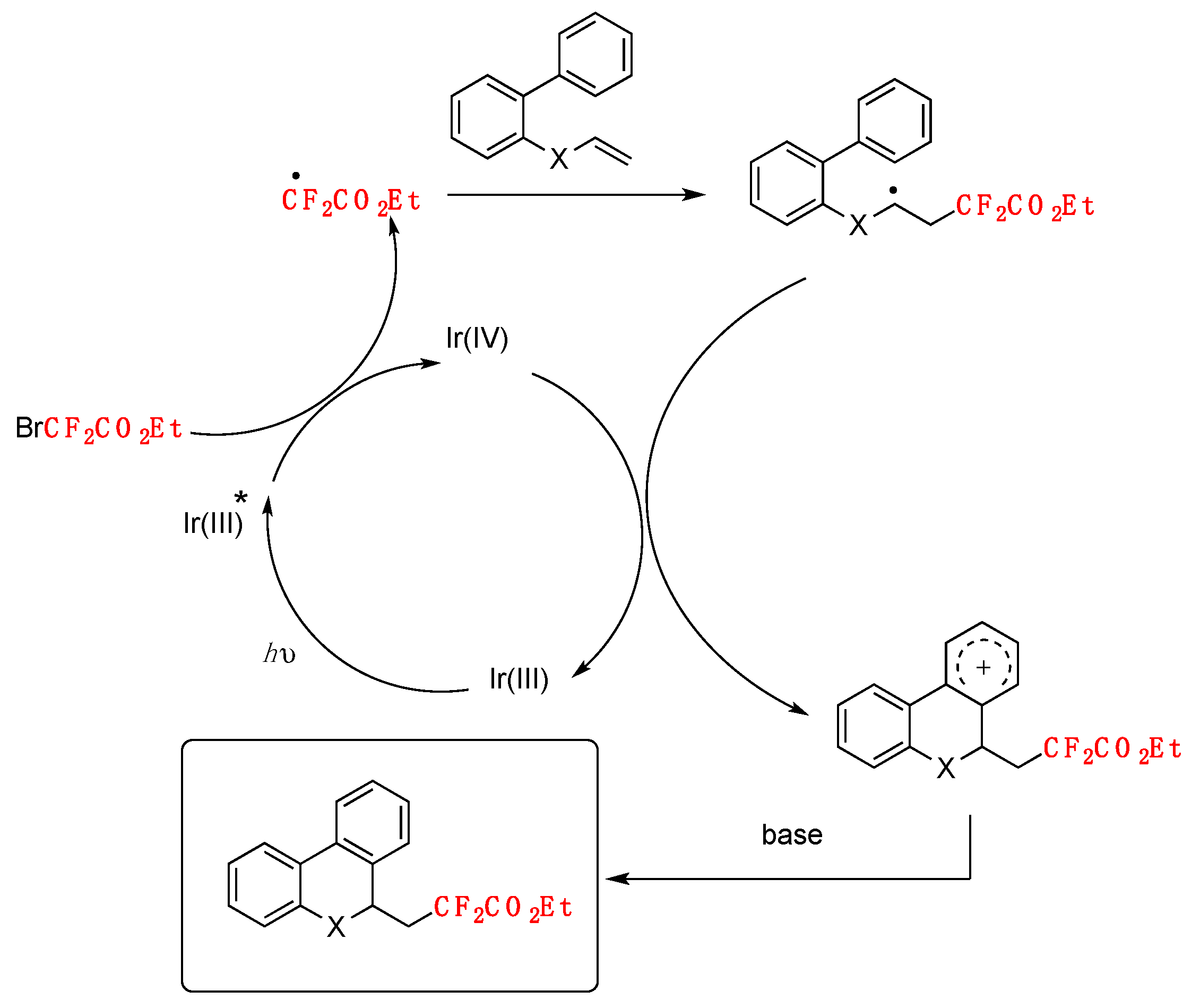
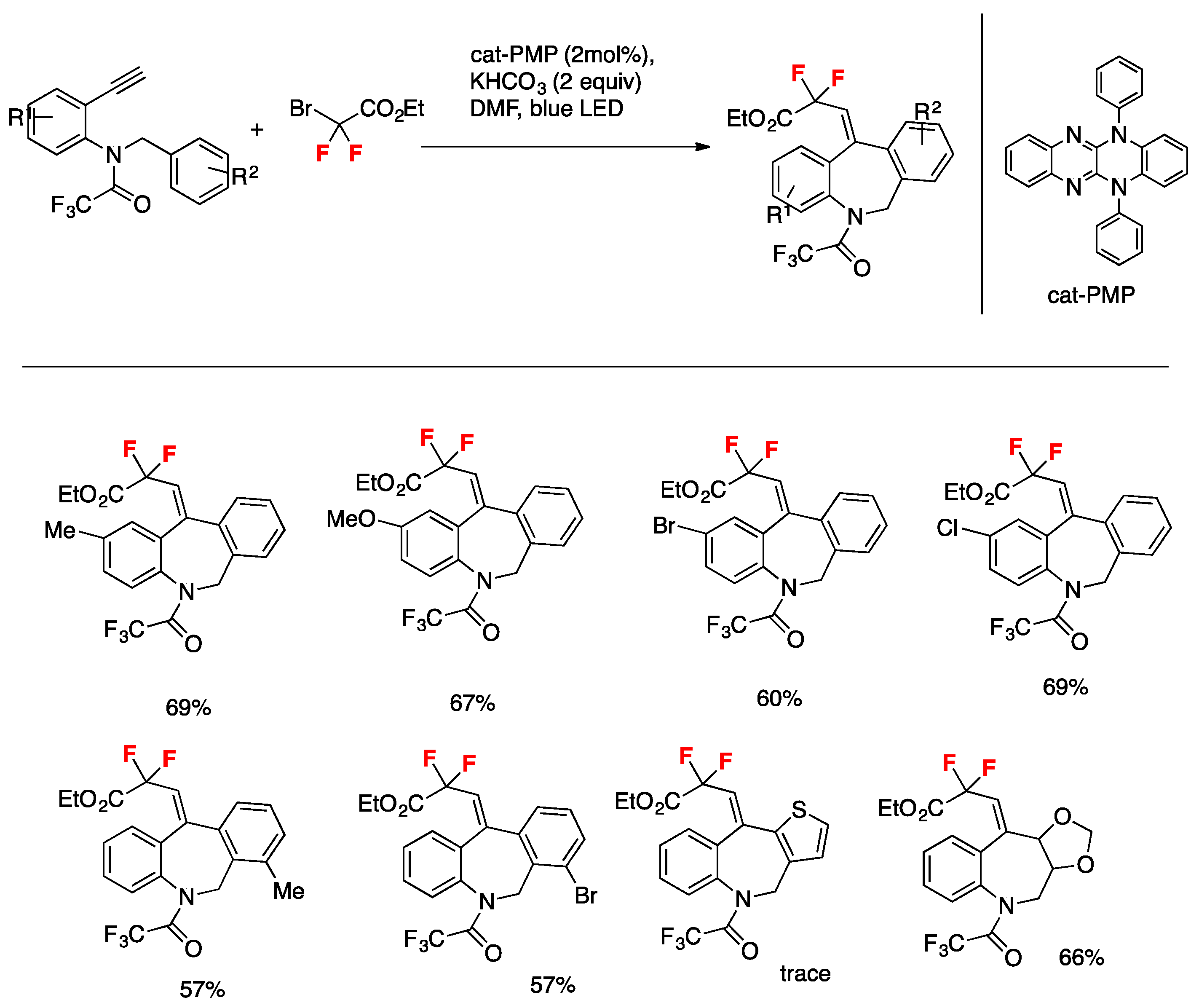
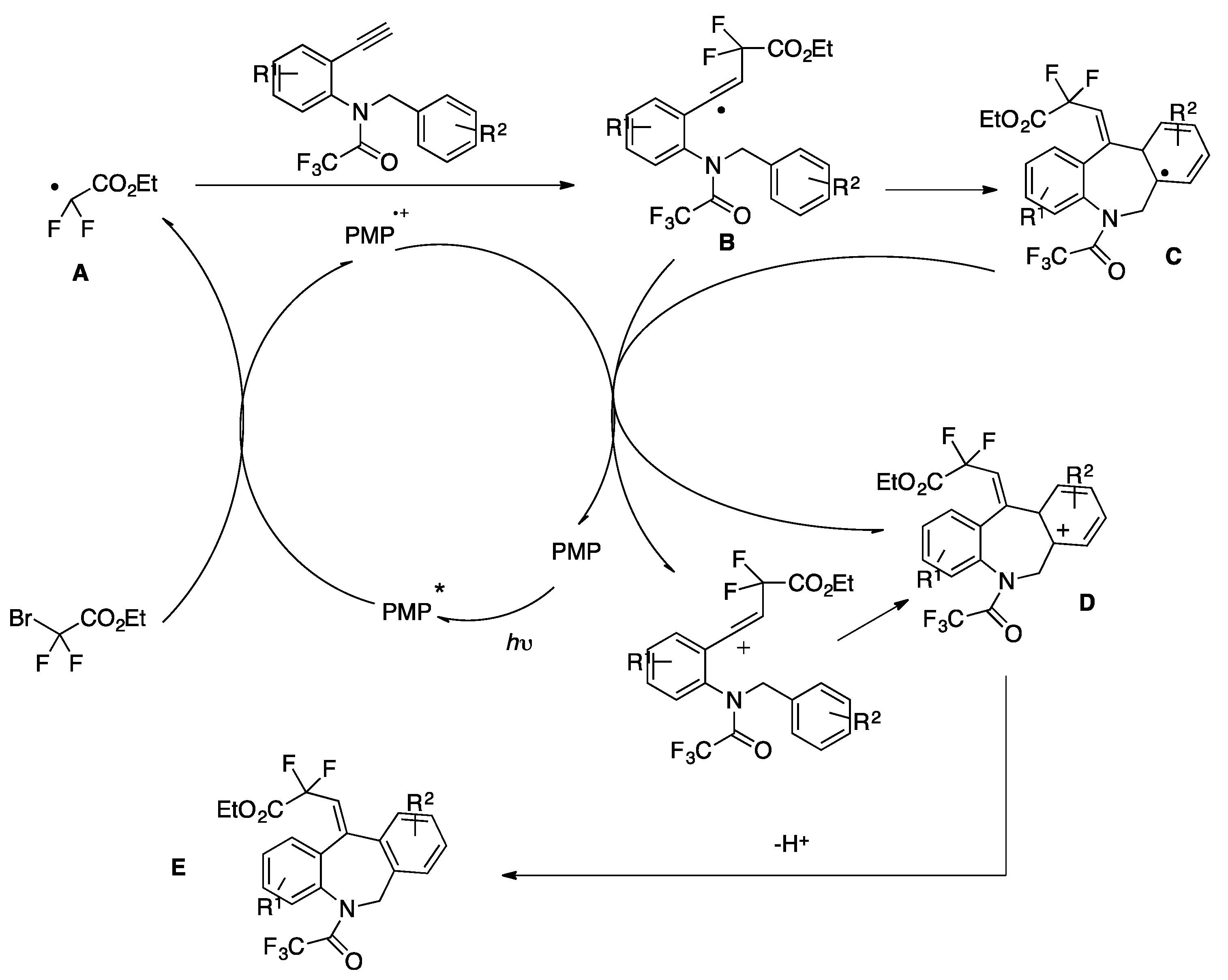
- Liu, D.; Jiao, M.-J.; Wang, X.-Z.; Xu, P.-F. Metal-Free Visible-Light-Induced Construction of Difluoro- Containing Dibenzazepines. Org. Lett. 2019, 21, 4745–4749. [Google Scholar] [CrossRef]
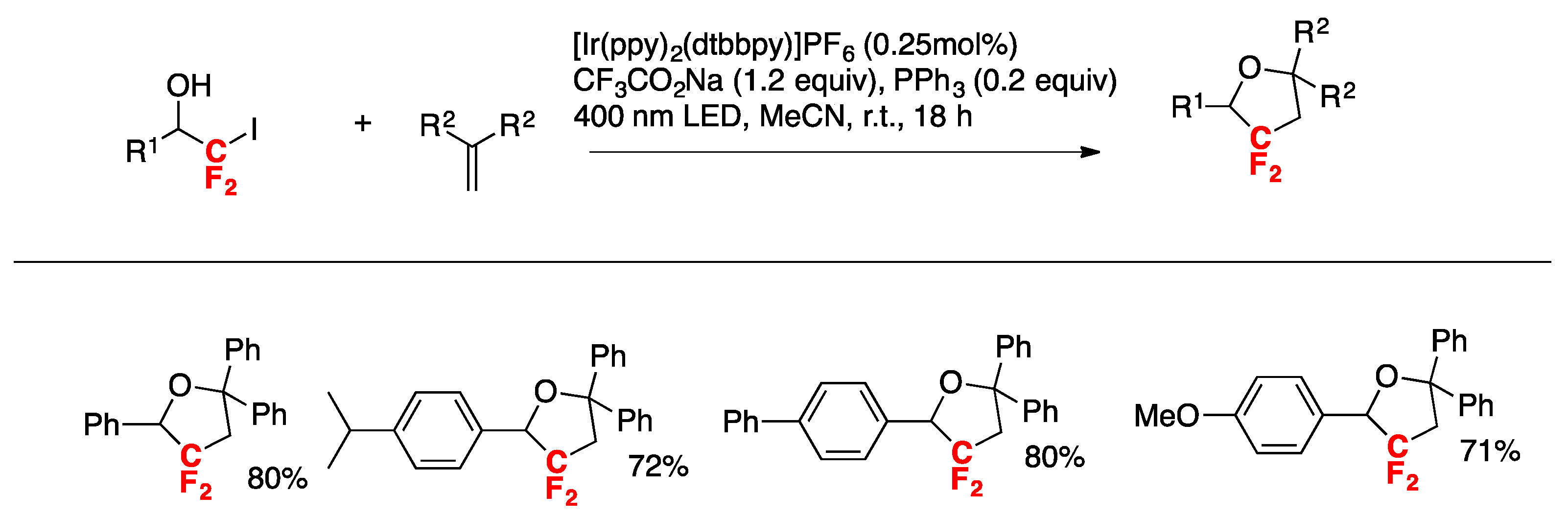
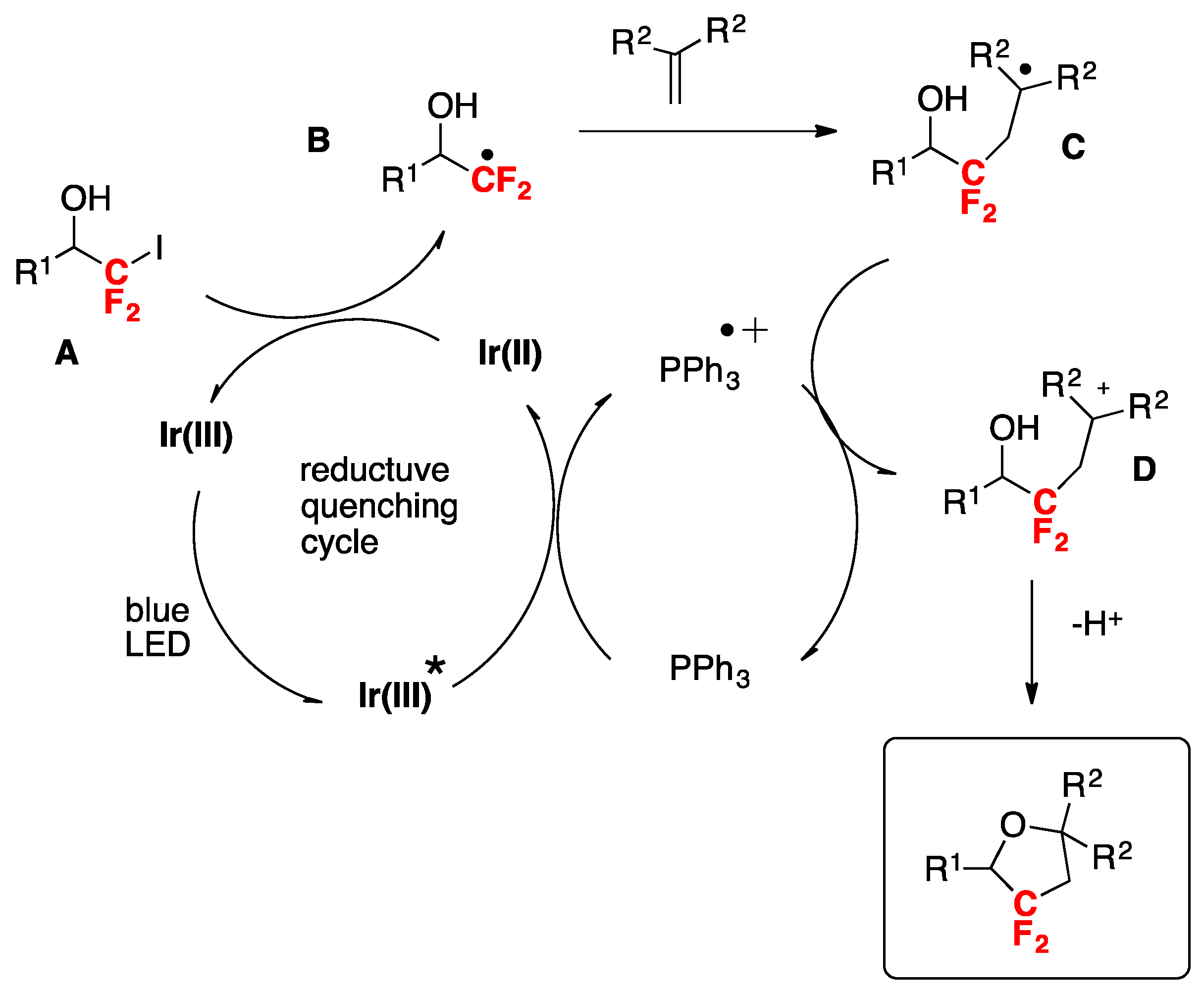
- Panferova, L.I.; Chernov, G.N.; Levin, V.V.; Kokorekin, V.A.; Dilman, A.D. Photoredox mediated annelation of iododifluoromethylated alcohols with 1,1-diaryethylenes. Tetrahedron 2018, 74, 7136–7142. [Google Scholar] [CrossRef]
- Wei, X.-J.; Noel, T. Visible-Light Photocatalytic Difluoroalkylation-Induced 1,2-Heteroarene Migration of Allylic Alcohols in Batch and Flow. J. Org. Chem. 2018, 83, 11377–11384. [Google Scholar] [CrossRef] [PubMed]
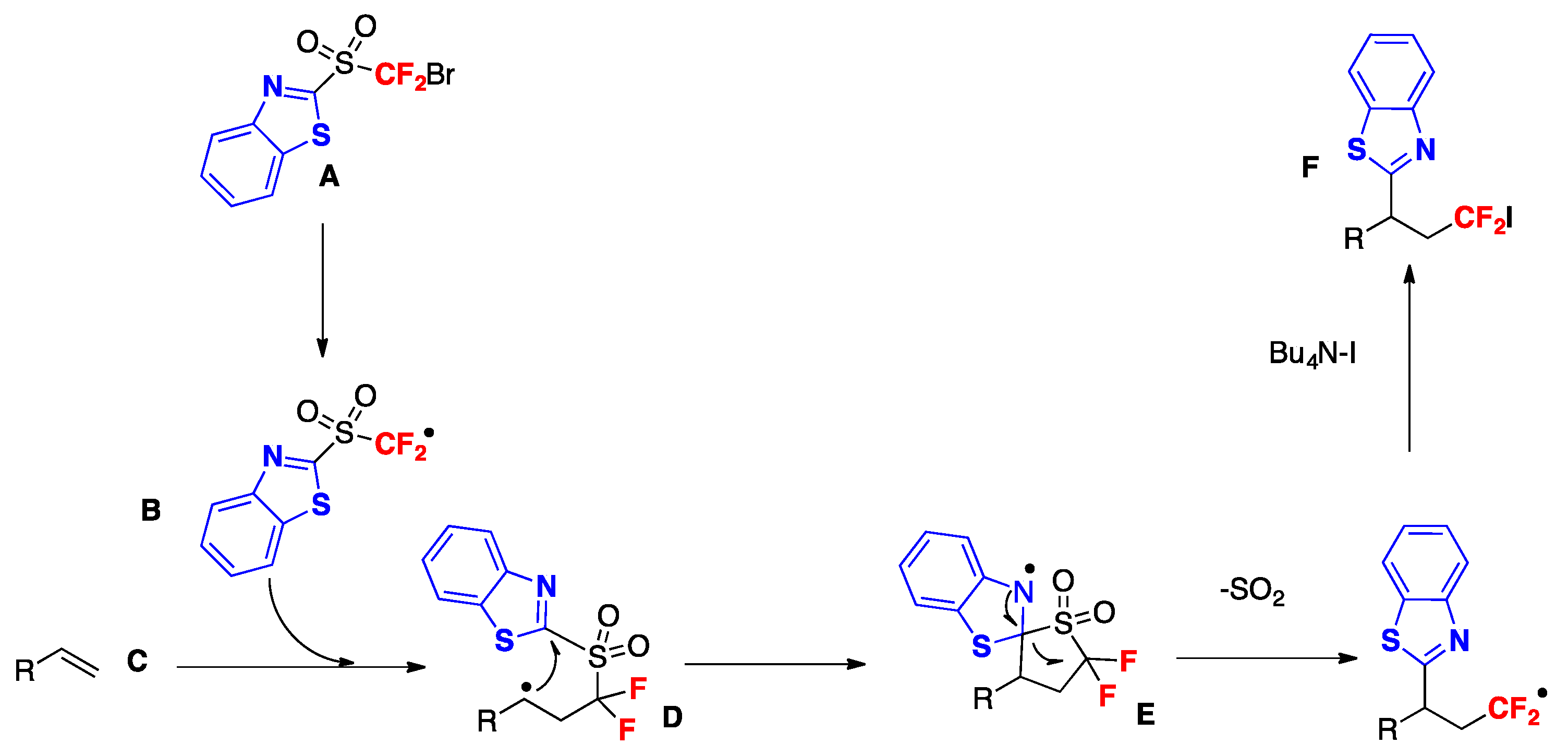
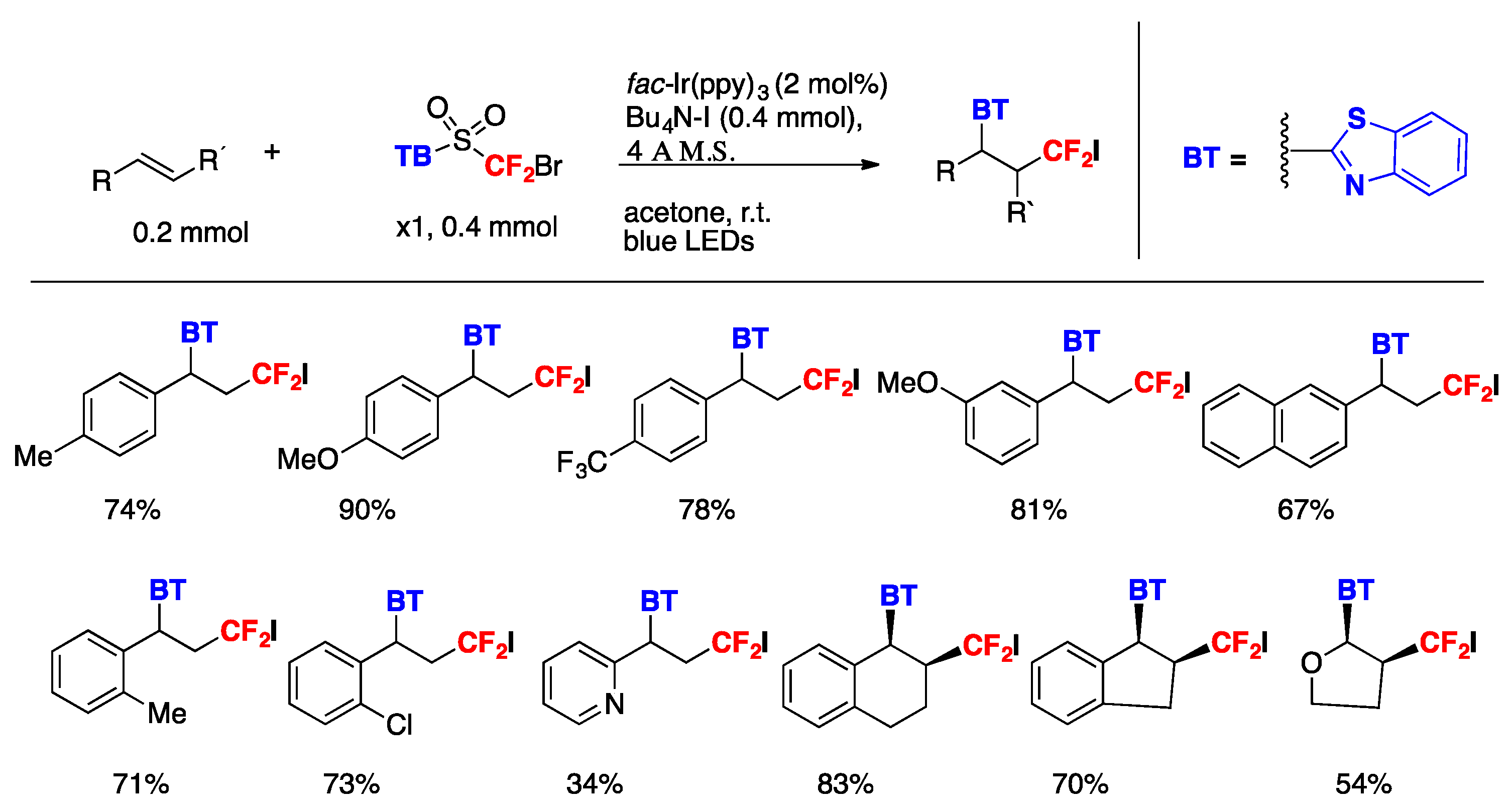
- Yu, J.; Wu, Z.; Zhu, C. Efficient Docking-Migration Strategy for Selective Radical Difluoromethylation of Alkenes. Angew. Chem. Int. Ed. 2018, 57, 17156–17160. [Google Scholar] [CrossRef] [PubMed]
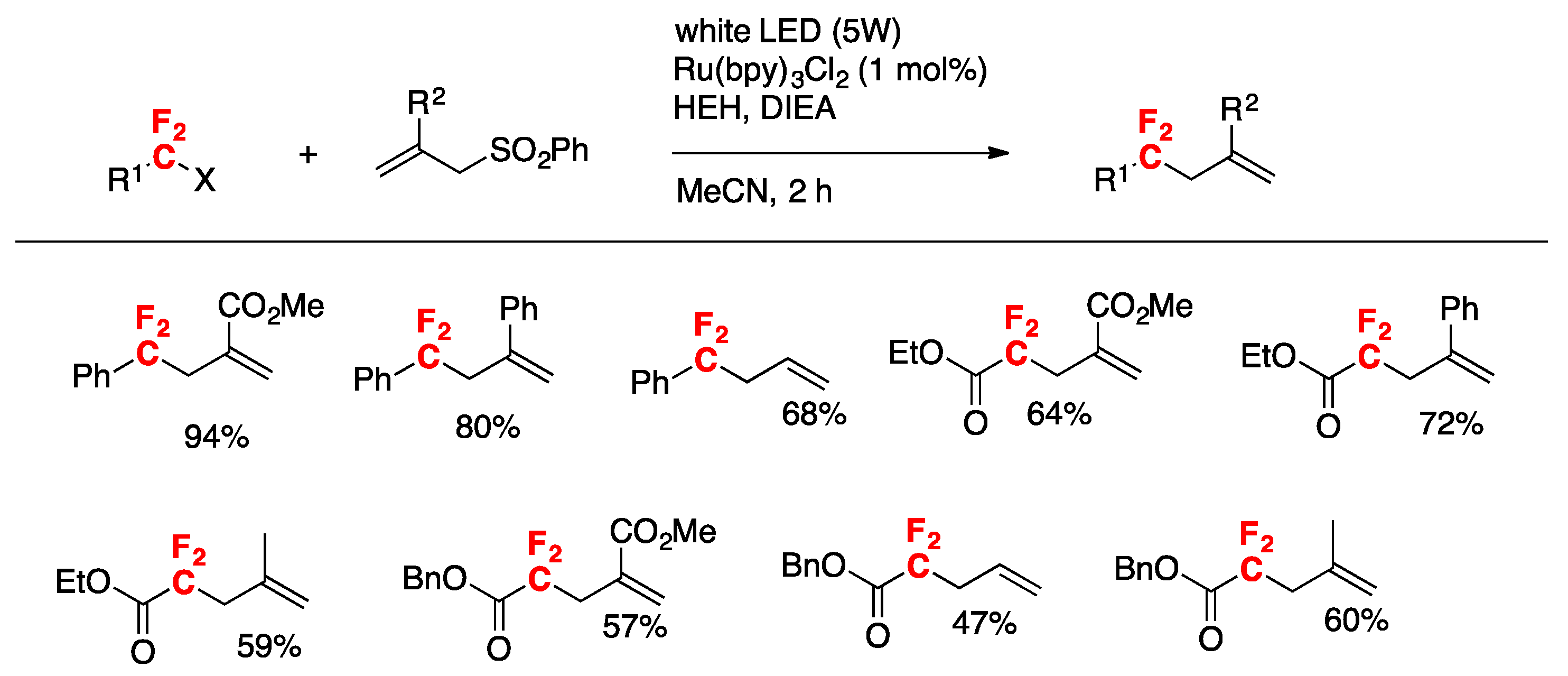
- Uno, M.; Sumino, S.; Fukuyama, T.; Matsuura, M.; Kuroki, Y.; Kishikawa, Y.; Ryu, I. Synthesis of 4,4-Difluoroalkenes by Coupling of α-Substituted α,α- Difluoromethyl Halides with Allyl Sulfones under Photoredox Catalyzed Conditions. J. Org. Chem. 2019, 84, 9330–9338. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, W.; Zhu, C. Photoredox 1,2-dicarbofunctionalization of Unactivated Alkenes via Tandem radical Difluoroalkylation and Alkynyl Migration. Org. Chem. Front. 2018, 5, 797–800. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Luo, Y.-C.; Wang, Z.-Y.; Xu, P.-F. A new approach to access difluoroalkylated diarylmethanes via visible light photocatalytic cross-coupling reactions. Chem. Commun. 2018, 54, 3993–3996. [Google Scholar] [CrossRef] [PubMed]

















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barata-Vallejo, S.; Postigo, A. Photocatalytic Difluoromethylation Reactions of Aromatic Compounds and Aliphatic Multiple C–C Bonds. Molecules 2019, 24, 4483. https://doi.org/10.3390/molecules24244483
Barata-Vallejo S, Postigo A. Photocatalytic Difluoromethylation Reactions of Aromatic Compounds and Aliphatic Multiple C–C Bonds. Molecules. 2019; 24(24):4483. https://doi.org/10.3390/molecules24244483
Chicago/Turabian StyleBarata-Vallejo, Sebastián, and Al Postigo. 2019. "Photocatalytic Difluoromethylation Reactions of Aromatic Compounds and Aliphatic Multiple C–C Bonds" Molecules 24, no. 24: 4483. https://doi.org/10.3390/molecules24244483