Synthesis and Novel Purification Process of PANI and PANI/AgNPs Composite
Abstract
:1. Introduction
2. Results and Discussion
2.1. PANI Characterization
2.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
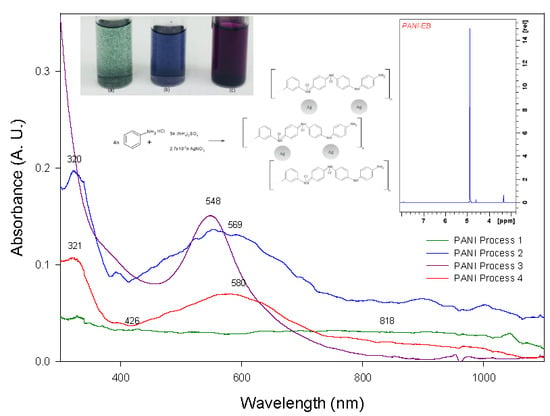
2.1.2. UV-Visible Spectroscopy (UV-Vis)
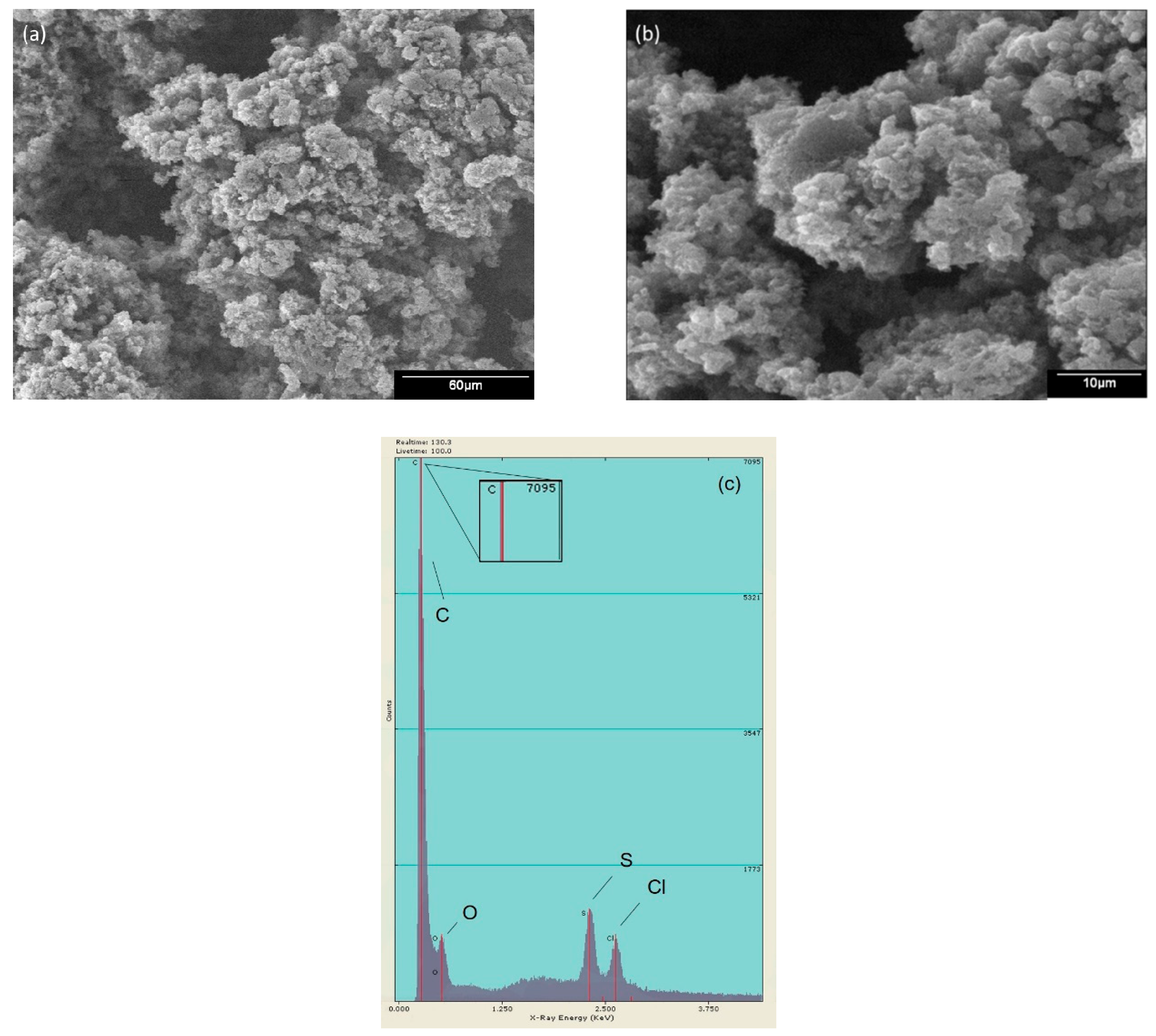
2.1.3. Scanning Electron Microscopy (SEM) and Electron Dispersive Energy (EDS)
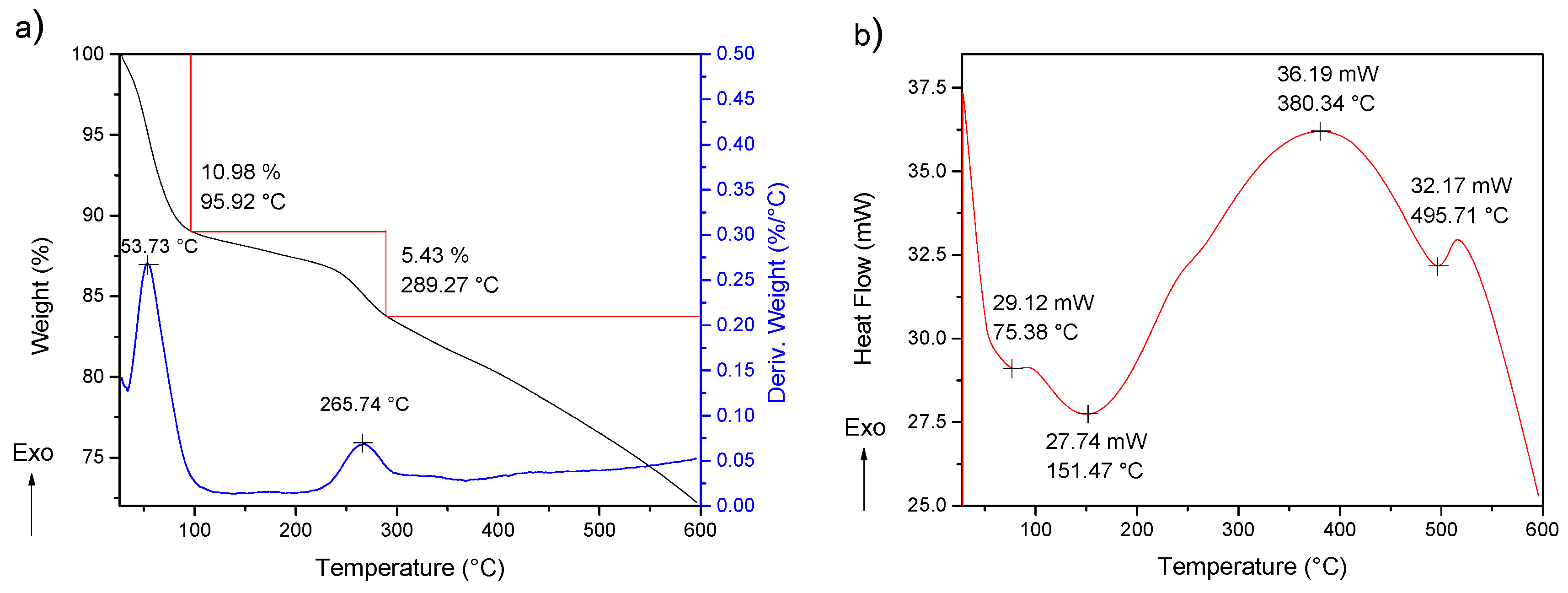
2.1.4. Thermogravimetric Analysis and Differential Scanning Calorimetry (TGA-DSC)
2.2. PANI/AgNPs Composite Characterization
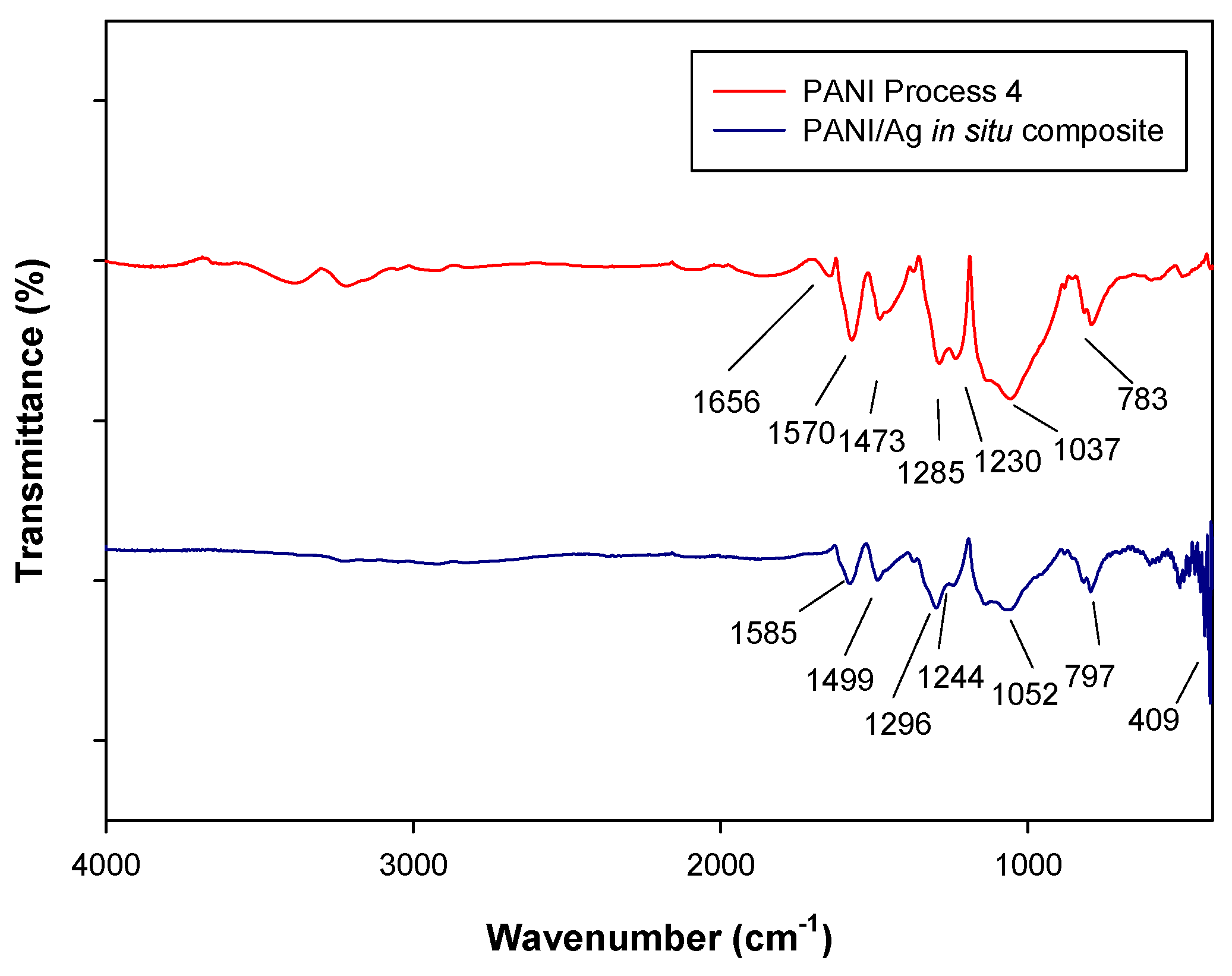
2.2.1. Fourier Transform Infrared Spectroscopy (FTIR)
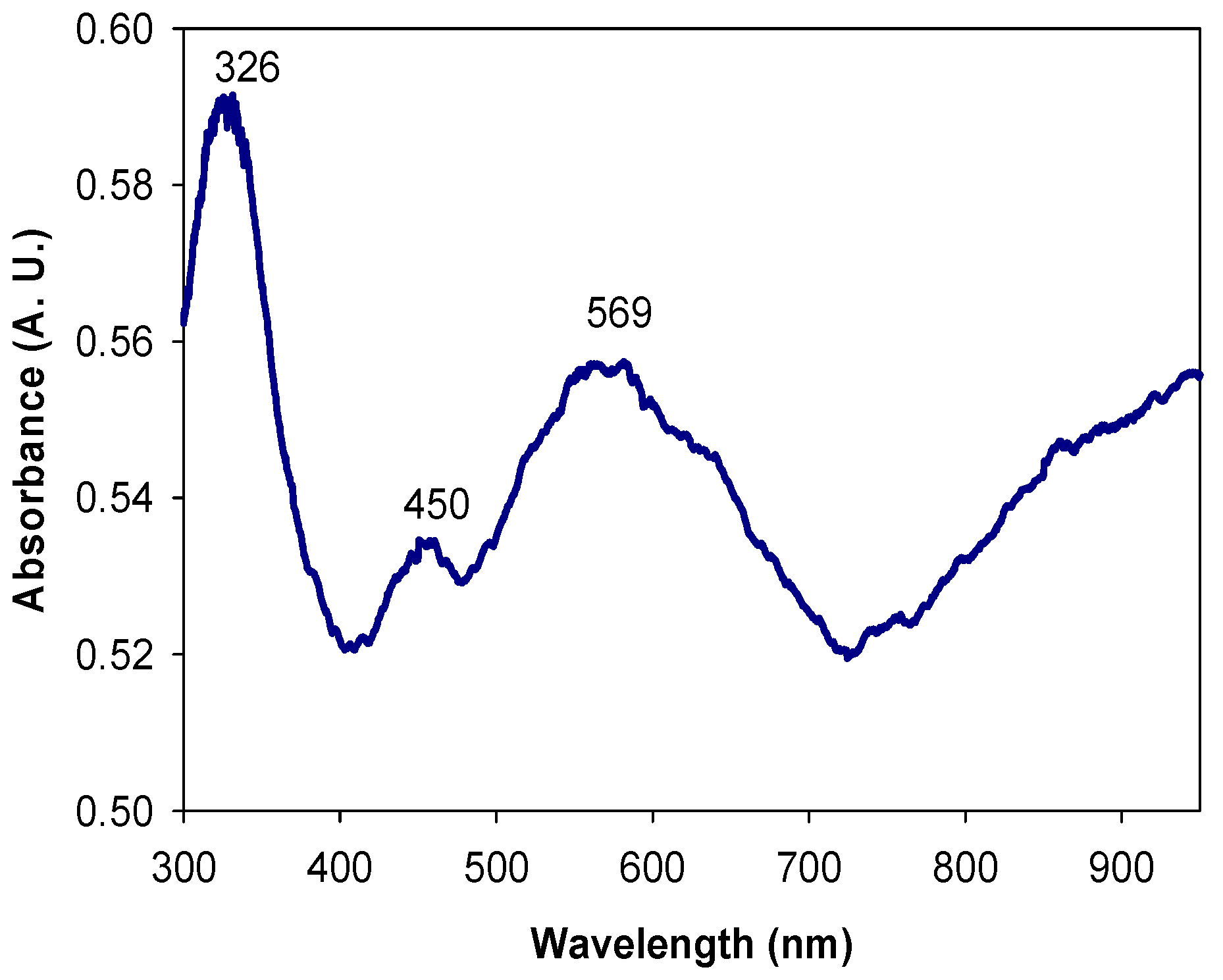
2.2.2. UV-Visible Spectroscopy (UV-Vis)
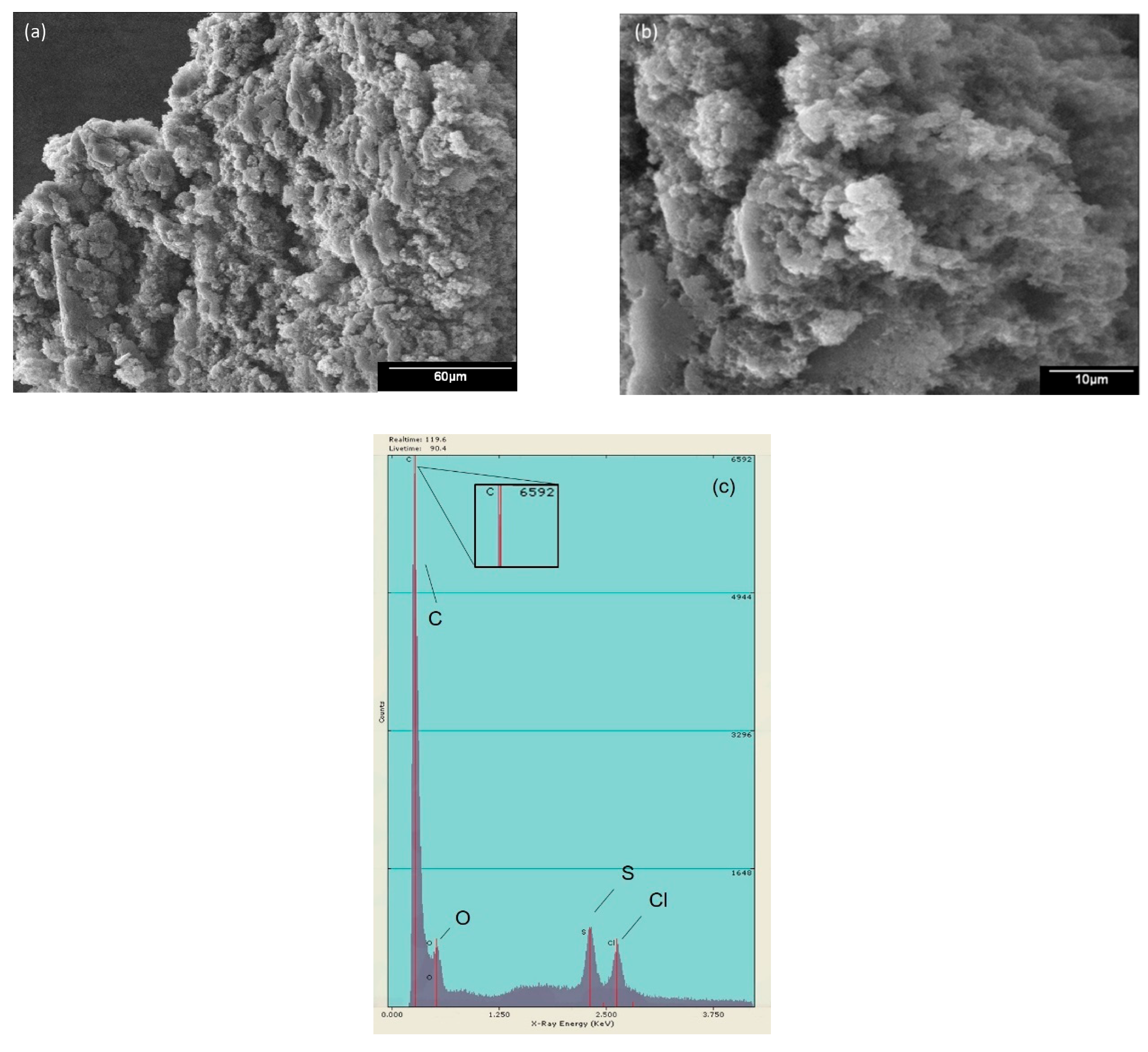
2.2.3. Scanning Electron Microscopy (SEM) and Electron Dispersive Energy (EDS)
2.2.4. Thermogravimetric Analysis and Differential Scanning Calorimetry (TGA-DSC)
3. Materials and Methods
3.1. Materials
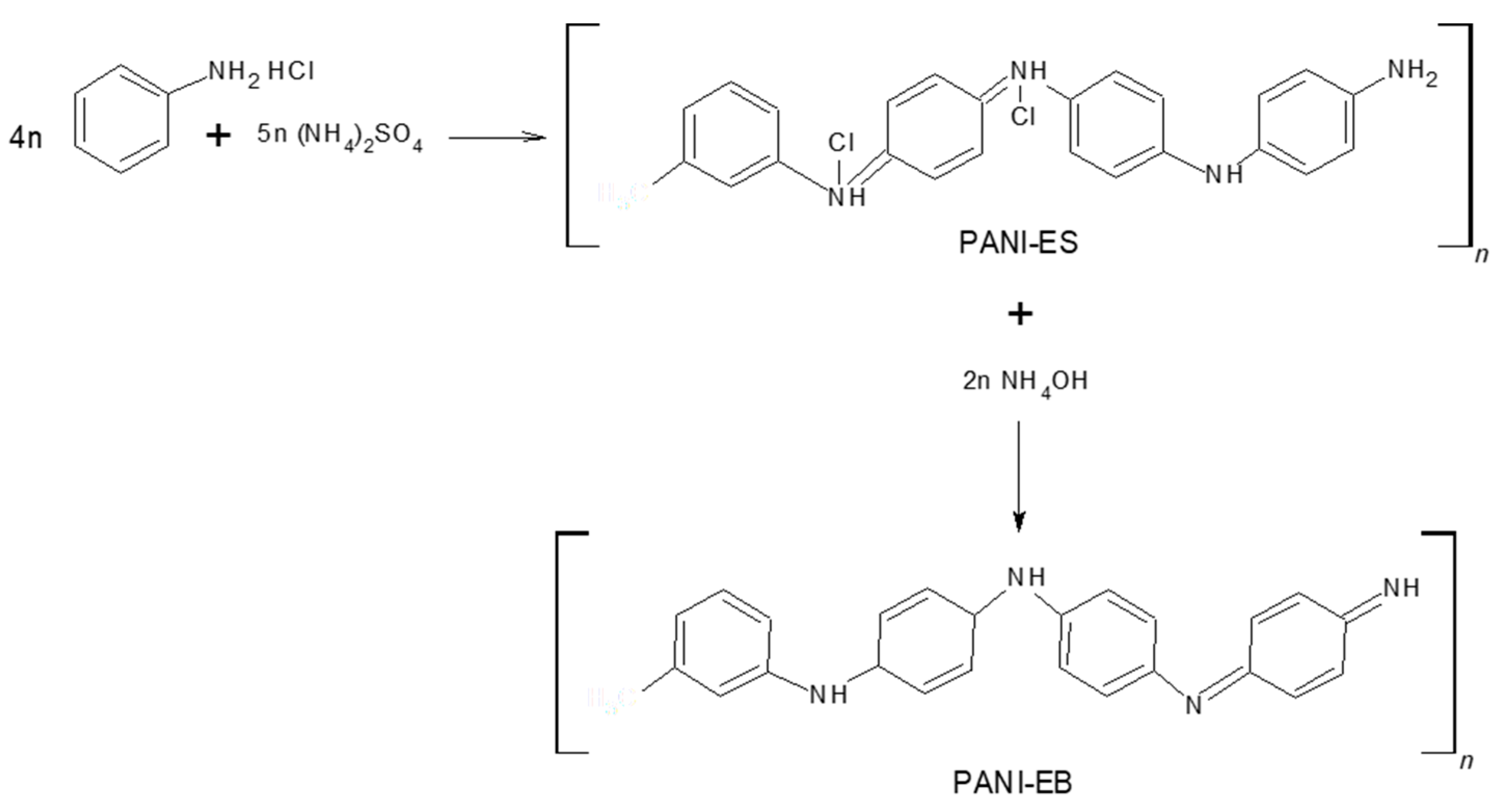
3.2. Polymerization of Aniline
3.3. Purification Process of PANI
3.3.1. Process 1
3.3.2. Process 2
3.3.3. Process 3
3.3.4. Process 4
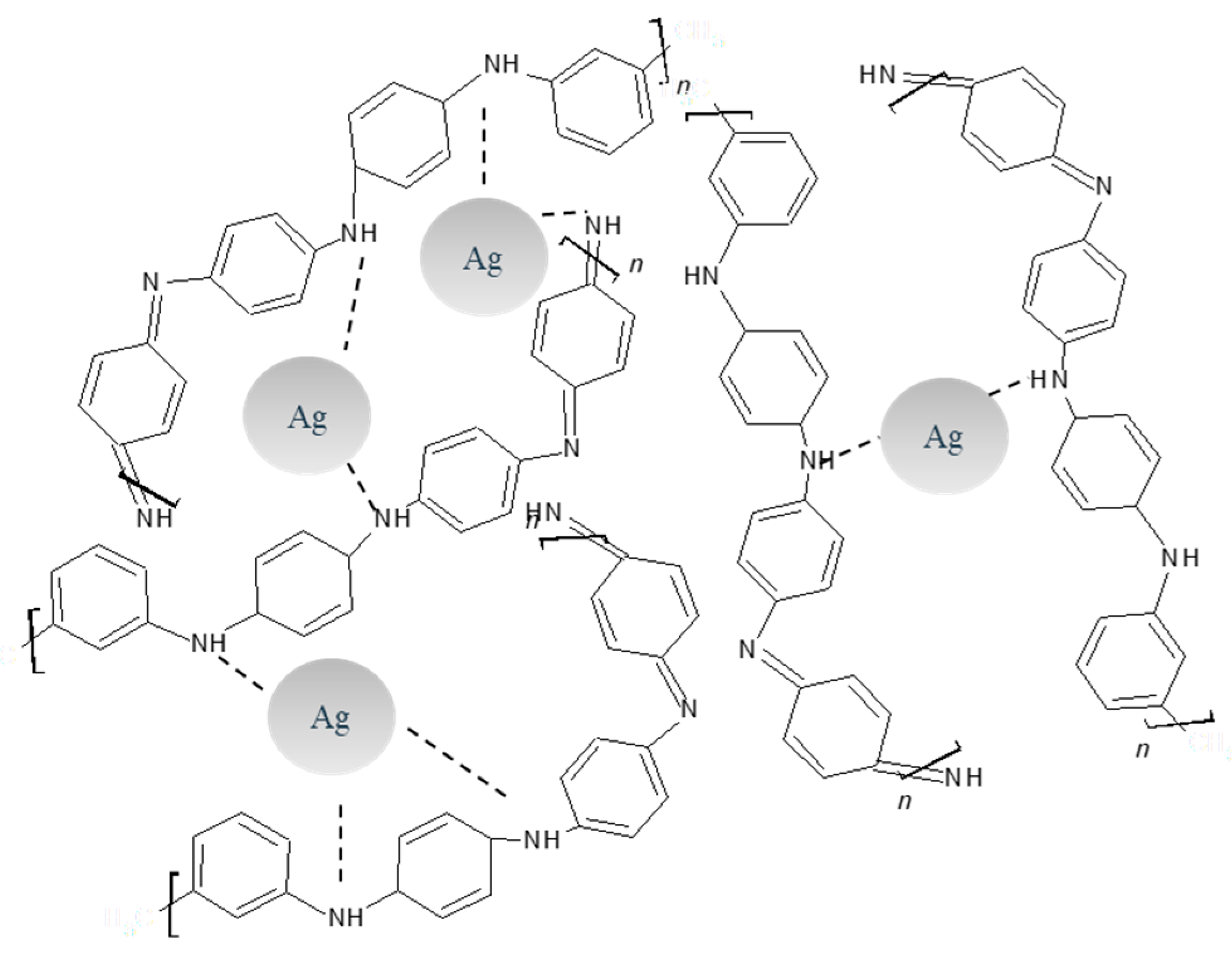
3.4. Synthesis of PANI/AgNPs in Situ Composite
3.5. Theorical Prediction of Surface Plasmon Resonance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pringsheim, E.; Terpetschnig, E.; Wolfbeis, O.S. Optical sensing of pH using thin films of substituted polyanilines. Anal. Chimica Acta 1997, 357, 247–252. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Geng, Y.H.; Sun, Z.C.; Li, J.; Jing, X.B.; Wang, X.H.; Wang, F.S. Water soluble polyaniline and its blend films prepared by aqueous solution casting. Polymer 1999, 40, 5723–5727. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y. Synthesis and applications of one-dimensional nano-structured polyaniline: An overview. Mat. Sci. Eng. B 2006, 134, 9–19. [Google Scholar] [CrossRef]
- Jaymand, M. Recent progress in chemical modification of polyaniline. Prog. Polym. Sci. 2013, 38, 1287–1306. [Google Scholar] [CrossRef]
- Chowdhury, A.-N. Trends in Polyaniline Research; Nova Science Publishers, Inc.: New York, NY, USA, 2013; ISBN 9781628084245. [Google Scholar]
- Wei, D.; Ivaska, A. Electrochemical biosensors based on polyaniline. Chem. Anal. (Warsaw) 2006, 51, 839–852. [Google Scholar]
- Wang, H.; Huang, E.L.; Gong, M.; Liu, D.; Wang, L. Synthesis, Characterization and Anticorrosion Performances Study of Different Acid Doping Polyaniline. Appl. Mec. Mat. 2014, 633–634, 246–249. [Google Scholar] [CrossRef]
- Dhand, C.; Das, M.; Datta, M.; Malhotra, B.D. Recent advances in polyaniline based biosensors. Bios. Bioel. 2011, 26, 2811–2821. [Google Scholar] [CrossRef]
- De Albuquerque, J.E.; Mattoso, L.H.C.; Faria, R.M.; Masters, J.G.; MacDiarmid, A.G. Study of the interconversion of polyaniline oxidation states by optical absorption spectroscopy. Synth. Met. 2004, 146, 1–10. [Google Scholar] [CrossRef]
- Dispenza, C.; Leone, M.; Presti, C.L.; Librizzi, F.; Spadaro, G.; Vetri, V. Optical properties of biocompatible polyaniline nano-composites. J. Non-Crystal. Solids 2006, 352, 3835–3840. [Google Scholar] [CrossRef]
- Tang, S.-J.; Wang, A.-T.; Lin, S.-Y.; Huang, K.-Y.; Yang, C.-C.; Yeh, J.-M.; Chiu, K.-C. Polymerization of aniline under various concentrations of APS and HCl. Polym. J. 2011, 43, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Tahir, Z.M.; Alocilja, E.C.; Grooms, D.L. Polyaniline synthesis and its biosensor application. Bios. Bioel. 2005, 20, 1690–1695. [Google Scholar] [CrossRef]
- Spence, D.; Polizos, G.; Joshi, P.; Sharma, J. A novel strategy to purify conductive polymer particles. RSC Adv. 2019, 9, 4857–4861. [Google Scholar] [CrossRef]
- Kašpárková, V.; Humpolíček, P.; Stejskal, J.; Kopecká, J.; Kuceková, Z.; Moučka, R. Conductivity, impurity profile, and cytotoxicity of solvent-extracted polyaniline. Poly. Adv. Technol. 2016, 27, 156–161. [Google Scholar] [CrossRef]
- Guerrero, J.M.; Carrillo, A.; Mota, M.L.; Ambrosio, R.C.; Aguirre, F.S. Purification and glutaraldehyde activation study on HCl-doped PVA-PANI copolymers with different aniline concentrations. Molecules 2019, 24, 63. [Google Scholar] [CrossRef]
- Mathai, C.J.; Saravanan, S.; Anantharaman, M.R.; Venkitachalam, S.; Jayalekshmi, S. Effect of iodine doping on the bandgap of plasma polymerized aniline thin films. J. Phy. D 2002, 35, 2206–2210. [Google Scholar] [CrossRef]
- Jelmy, E.J.; Ramakrishnan, S.; Devanathan, S.; Rangarajan, M.; Kothurkar, N.K. Optimization of the conductivity and yield of chemically synthesized polyaniline using a design of experiments. J. Appl. Poly. Sci. 2013, 130, 1047–1057. [Google Scholar] [CrossRef]
- Oliveira, R.D.S.; Bizeto, M.A.; Camilo, F.F. Production of self-supported conductive films based on cellulose, polyaniline and silver nanoparticles. Carbohyd. Poly. 2018, 199, 84–91. [Google Scholar]
- Wang, Y.; Bian, L.; Tan, D.; Chen, S.; Gan, Y. Sonochemical synthesis of “sea-island” structure silver/polyaniline nanocomposites for the detection of l-tyrosine. J. Therm. Comp. Mat. 2017, 30, 1033–1044. [Google Scholar] [CrossRef]
- Bláha, M.; Trchová, M.; Bober, P.; Morávková, Z.; Zujovic, Z.D.; Filippov, S.K.; Prokeš, J.; Pilař, J.; Stejskal, J. Structure and properties of polyaniline interacting with H-phosphonates. Synth. Metals 2017, 232, 79–86. [Google Scholar] [CrossRef]
- Zhu, H.; Peng, S.; Jiang, W. Electrochemical properties of PANI as single electrode of electrochemical capacitors in acid electrolytes. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Bláha, M.; Trchová, M.; Bober, P.; Morávková, Z.; Prokeš, J.; Stejskal, J. Polyaniline: Aniline oxidation with strong and weak oxidants under various acidity. Mat. Chem. Phy. 2017, 194, 206–218. [Google Scholar] [CrossRef]
- Sun, Y.; MacDiarmid, A.G.; Epstein, A.J. Polyaniline—Synthesis and characterization of pernigraniline base. J. Chem. Soc. Chem. Commun. 1990, 529–531. [Google Scholar]
- Nabid, M.R.; Asadi, S.; Sedghi, R.; Bayandori Moghaddam, A. Chemical and enzymatic polymerization of polyaniline/ag nanocomposites. Chem. Eng. Technol. 2013, 36, 1411–1416. [Google Scholar] [CrossRef]
- Mishra, S.; Shimpi, N.G.; Sen, T. The effect of PEG encapsulated silver nanoparticles on the thermal and electrical property of sonochemically synthesized polyaniline/silver nanocomposite. J. Poly. Res. 2013, 20, 32–35. [Google Scholar] [CrossRef]
- Singh, R.P.; Tiwari, A.; Pandey, A.C. Silver/Polyaniline Nanocomposite for the Electrocatalytic Hydrazine Oxidation. J. Inorg. Org. Poly. Mat. 2011, 21, 788–792. [Google Scholar] [CrossRef] [Green Version]
- Bedre, M.D.; Basavaraja, S.; Salwe, B.D.; Shivakumar, V.; Arunkumar, L.; Venkataraman, A. Preparation and characterization of Pani and Pani-Ag nanocomposites via interfacial polymerization. Poly. Comp. 2009, 30, 1668–1677. [Google Scholar] [CrossRef]
- Khan, M.J.; Husain, Q.; Ansari, S.A. Polyaniline-assisted silver nanoparticles: A novel support for the immobilization of α-amylase. Appl. Microbiol. Biotechnol. 2013, 97, 1513–1522. [Google Scholar] [CrossRef]
- Petrovski, A.; Paunović, P.; Avolio, R.; Errico, M.E.; Cocca, M.; Gentile, G.; Grozdanov, A.; Avella, M.; Barton, J.; Dimitrov, A. Synthesis and characterization of nanocomposites based on PANI and carbon nanostructures prepared by electropolymerization. Mat. Chem. Phy. 2017, 185, 83–90. [Google Scholar] [CrossRef]
- Gupta, K.; Jana, P.C.; Meikap, A.K. Optical and electrical transport properties of polyaniline-silver nanocomposite. Synth. Metals 2010, 160, 1566–1573. [Google Scholar] [CrossRef]
- Ran, F.; Tan, Y.; Dong, W.; Liu, Z.; Kong, L.; Kang, L. In situ polymerization and reduction to fabricate gold nanoparticle-incorporated polyaniline as supercapacitor electrode materials. Poly. Adv. Technol. 2018, 29, 1697–1705. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
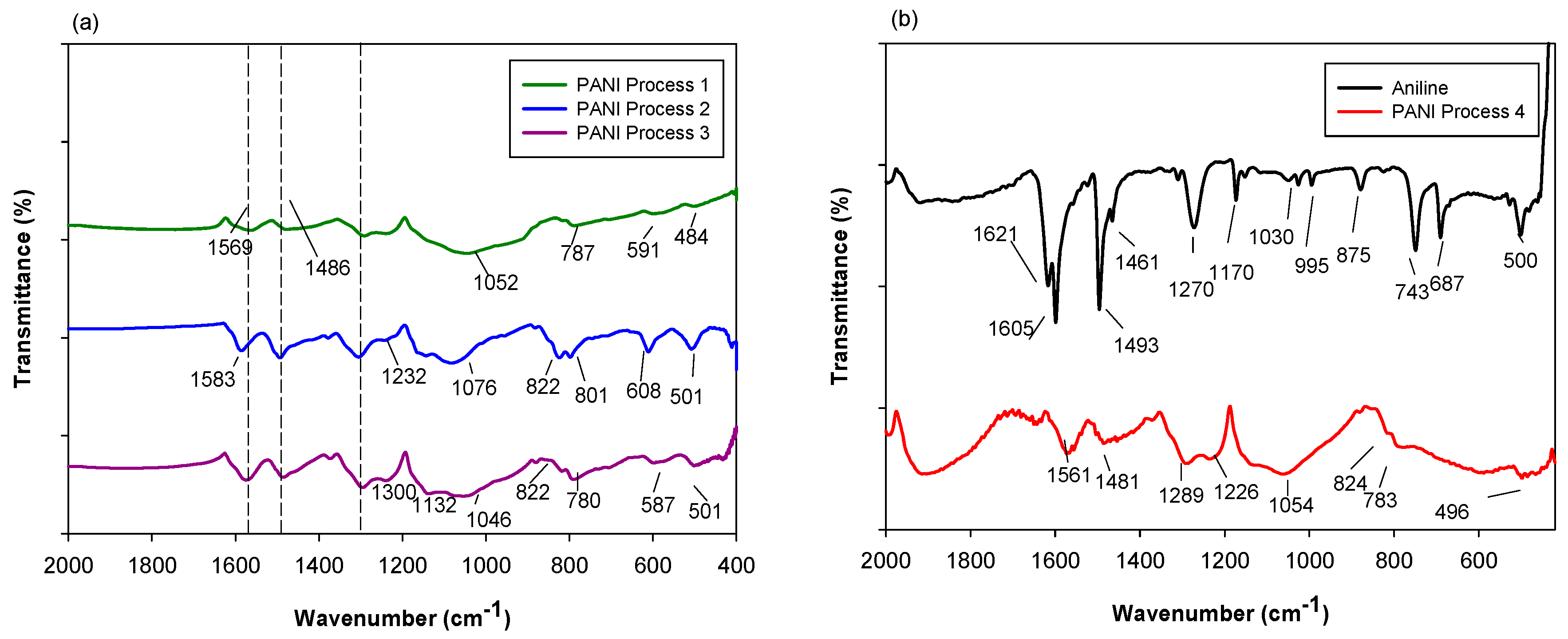



| PANI P1 (cm−1) | PANI P2 (cm−1) | PANI P3 (cm−1) | PANI P4 (cm−1) | Assignment |
|---|---|---|---|---|
| 1569 | 1583 | 1569 | 1561 | Quinoid ring stretching |
| 1486 | 1486 | 1486 | 1481 | Benzenoid ring stretching |
| - | 1378 | 1373 | 1371 | C–N stretching vibration near quinonediimine unit |
| 1300 | 1300 | 1300 | 1289 | C–N stretching in cis-Q-B-Q 1, Q-B-B 2 and B-B-Q 3 |
| 1232 | 1232 | 1232 | 1226 | C–N stretching in B-B-B 4 |
| 1052 | 1076 | 1046 | 1054 | |
| - | 822 | 822 | 824 | C–H out of plane bending of 1,2,4-ring |
| 787 | 801 | 780 | 783 | |
| 591 | 608 | 587 | - | |
| 484 | 501 | 501 | 496 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, M.L.; Carrillo, A.; Verdugo, A.J.; Olivas, A.; Guerrero, J.M.; De la Cruz, E.C.; Noriega Ramírez, N. Synthesis and Novel Purification Process of PANI and PANI/AgNPs Composite. Molecules 2019, 24, 1621. https://doi.org/10.3390/molecules24081621
Mota ML, Carrillo A, Verdugo AJ, Olivas A, Guerrero JM, De la Cruz EC, Noriega Ramírez N. Synthesis and Novel Purification Process of PANI and PANI/AgNPs Composite. Molecules. 2019; 24(8):1621. https://doi.org/10.3390/molecules24081621
Chicago/Turabian StyleMota, María L., Amanda Carrillo, Ana J. Verdugo, Amelia Olivas, Jorge M. Guerrero, Edna C. De la Cruz, and Natalia Noriega Ramírez. 2019. "Synthesis and Novel Purification Process of PANI and PANI/AgNPs Composite" Molecules 24, no. 8: 1621. https://doi.org/10.3390/molecules24081621