Novel Carbon/PEDOT/PSS-Based Screen-Printed Biosensors for Acetylcholine Neurotransmitter and Acetylcholinesterase Detection in Human Serum
Abstract
:1. Introduction
2. Results and Discussion
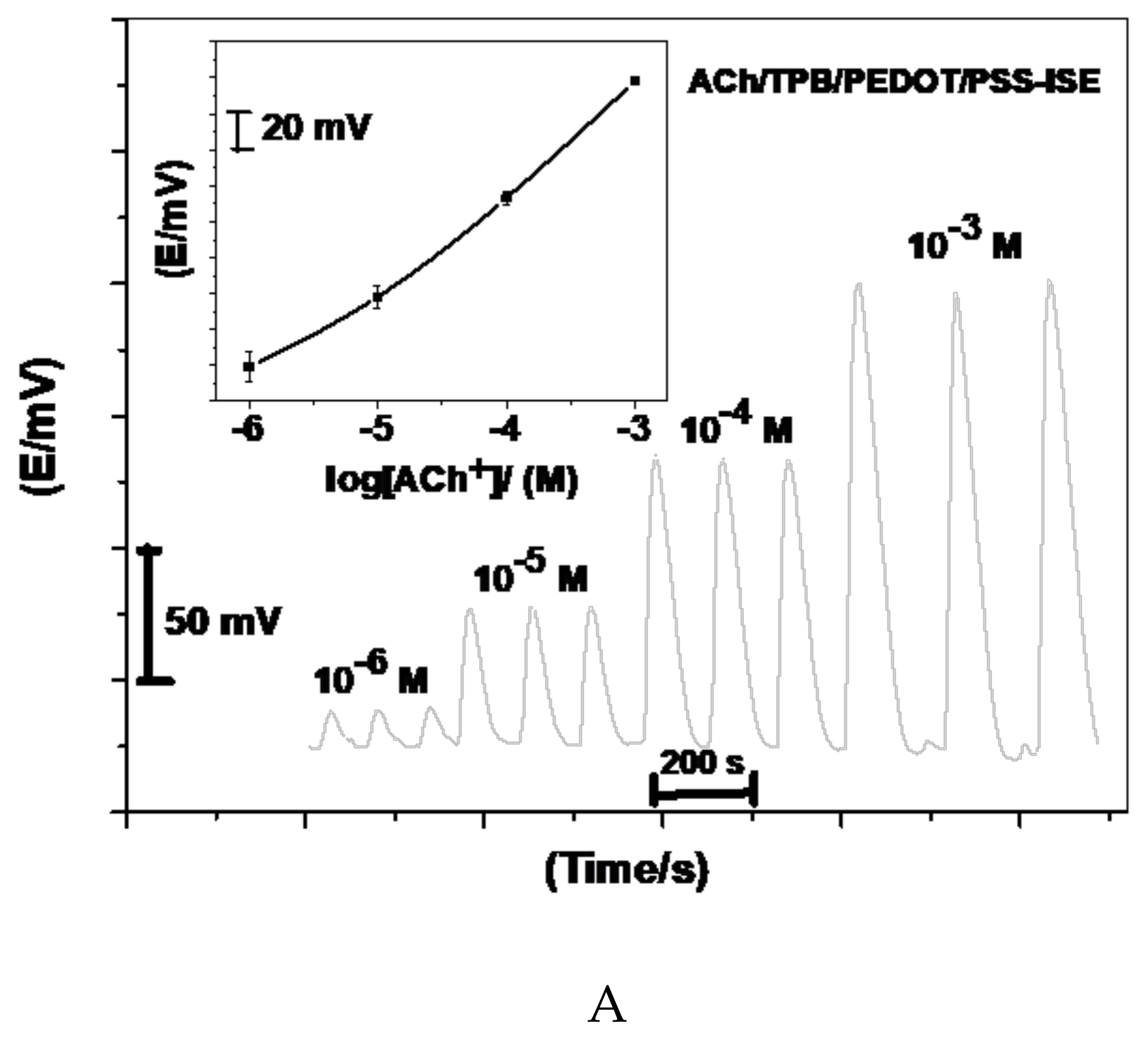
2.1. Sensors Performance Characteristics
2.2. Sensor Selectivity
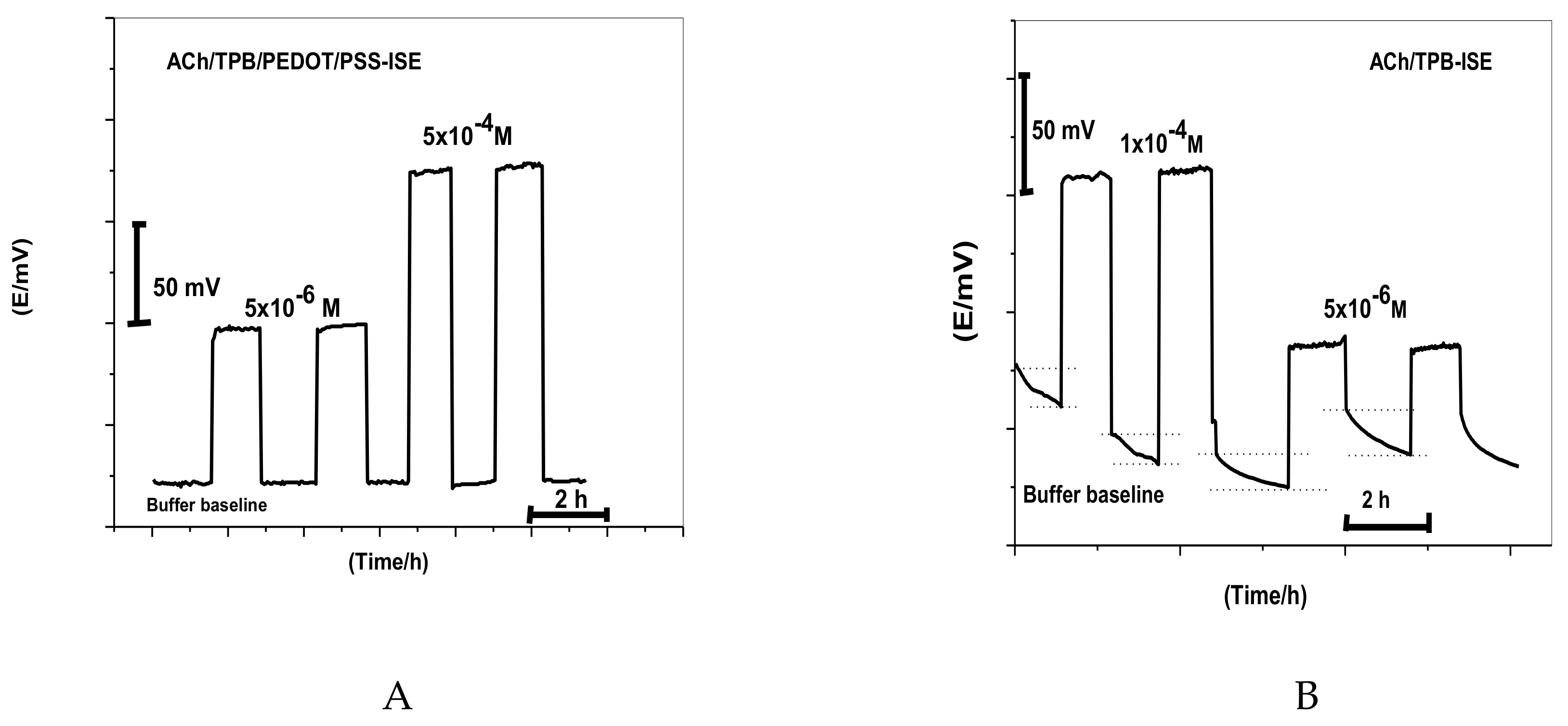
2.3. Effect of the PEDOT/PSS Solid-Contact Layer
2.4. Water-Layer Effect
2.5. Hydrodynamic Assessment of ACh+
2.6. Acetylcholine Assay in Human Serum
2.7. Kinetic Monitoring of Acetylcholinesterase Activity
3. Materials and Methods
3.1. Chemicals and Reagents
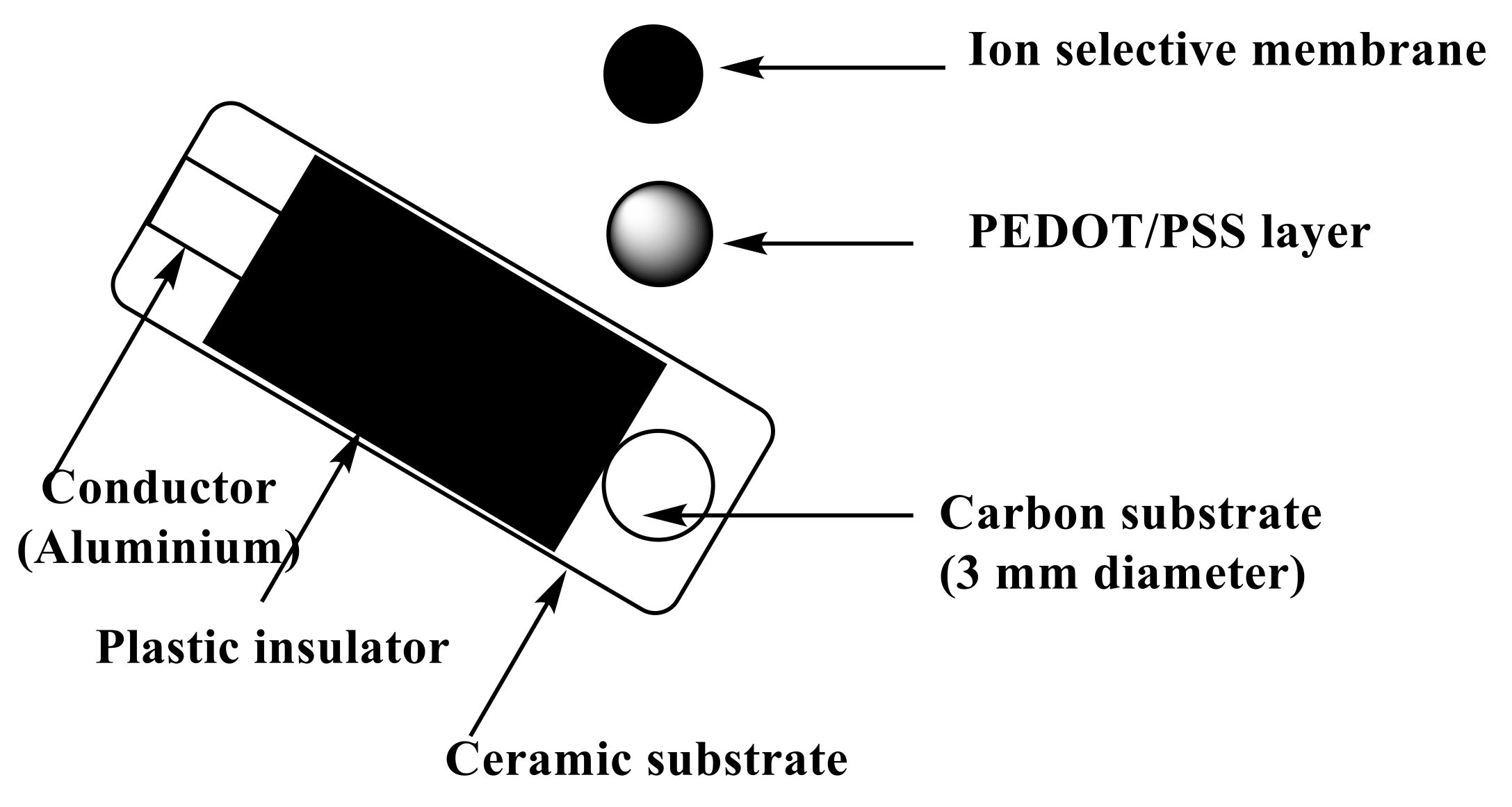
3.2. Apparatus
3.3. ISE Membranes and Electrodes Measurements
3.4. Acetylcholine Assay in Human Serum
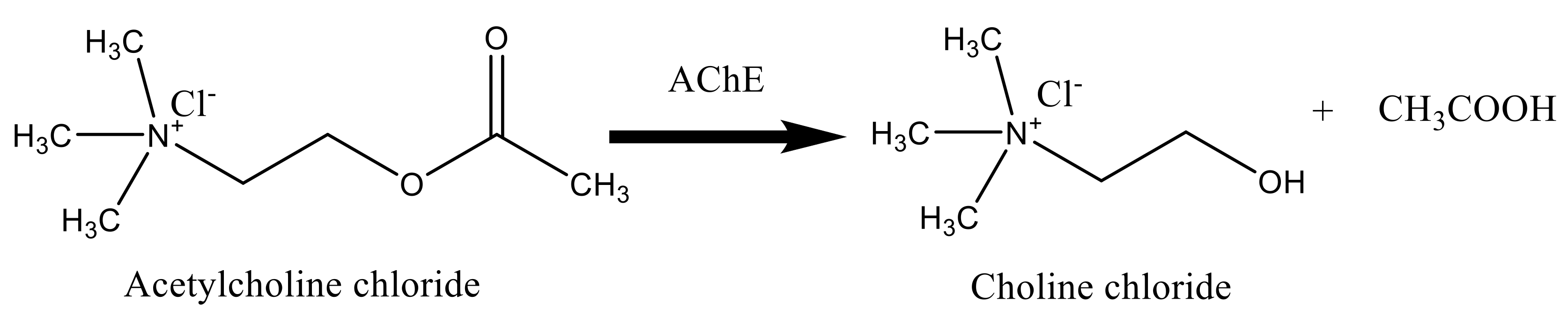
3.5. Bioassay of Acetyl Cholinesterase Enzyme (AChE)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics
References
- Moon, J.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Mesulam, M.M. Cholinergic Neurons, Pathways, Diseases. In Encyclopedia of Neuroscience; Adelman, G., Ed.; Birkhuser: Boston, MA, USA, 1987; Volume 1. [Google Scholar]
- Watanabe, M.; Kimura, A.; Akasaka, K.; Hayashi, S. Determination of acetylcholine in human blood. Biochem. Med. Metab. Biol. 1986, 36, 355–362. [Google Scholar] [CrossRef]
- Fraunfelder, F.T.; Meyer, S.M. Drug-Induced Ocular Side Effects and Drug Interactions, 2nd ed.; Lea & Febiger: Philadelphia, PA, USA, 1982. [Google Scholar]
- Coulson, F.R.; Fryer, A.D. Muscarinic acetylcholine receptors and airway diseases. Pharmacol. Ther. 2003, 98, 59–69. [Google Scholar] [CrossRef]
- Khayyal, M.T.; Tolba, H.M.; El-Hawary, M.B.; El-Wahed, S.A. A sensitive method for the bioassay of acetylcholine. Eur. J. Pharmacol. 1974, 25, 287–290. [Google Scholar] [CrossRef]
- Feigenson, M.E.; Saelens, J.K. An enzyme assay for acetylcholine. Biochem. Pharmacol. 1969, 18, 1479–1486. [Google Scholar] [CrossRef]
- Potter, P.E.; Meek, J.K.; Neff, N.H. Acetylcholine and Choline in Neuronal Tissue Measured by HPLC with Electrochemical Detection. J. Neurochem. 1983, 41, 188–194. [Google Scholar] [CrossRef]
- Okuyama, S.; Ikeda, Y. Determination of acetylcholine and choline in human cerebrospinal fluid using high-performance liquid chromatography combined with an immobilized enzyme reactor: Ageing-induced change of acetylcholine level. J. Chromatogr. B 1983, 431, 389–394. [Google Scholar] [CrossRef]
- MacDonald, R.C. A fluorometric assay for acetylcholine with picomole sensitivity. J. Neurosci. Methods 1989, 29, 73–76. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, L.; Shangguan, D.; Zhao, R.; Liu, G. Improved method for the routine determination of acetylcholine and choline in brain microdialysate using a horseradish peroxidase column as the immobilized enzyme reactor. J. Chromatogr. B 2003, 788, 193–198. [Google Scholar] [CrossRef]
- Liberato, D.J.; Yergey, A.L.; Weintraub, S.T. Separation and quantification of choline and acetylcholine by thermospray liquid chromatography/mass spectrometry. Biomed. Environ. Mass Spectrom. 1986, 13, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, H.; Ikarashi, Y.; Maruyama, Y. Use of high-performance liquid chromatography continuous-flow fast atom bombardment mass spectrometry for simultaneous determination of choline and acetylcholine in rodent brain regions. Biol. Mass Spectrom. 1993, 22, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Hows, M.E.P.; Organ, A.J.; Murray, S.; Dawson, L.A.; Foxton, R.; Heidbreder, C.; Hughes, Z.A.; Lacroix, L.; Shah, A.J. High-performance liquid chromatography/tandem mass spectrometry assay for the rapid high sensitivity measurement of basal acetylcholine from microdialysates. J. Neurosci. Methods 2002, 121, 33–39. [Google Scholar] [CrossRef]
- Dunphy, R.; Burinsky, D.J. Detection of choline and acetylcholine in a pharmaceutical preparation using high-performance liquid chromatography/electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2003, 31, 905–915. [Google Scholar] [CrossRef]
- Kiba, N.; Ito, S.; Tachbana, M.; Tani, K.; Koizumi, H. Simultaneous determination of choline and acetylcholine based on a trienzyme chemiluminometric biosensor in a single line flow injection system. Anal. Sci. 2003, 19, 1647–1651. [Google Scholar] [CrossRef]
- Bundel, D.D.; Sarre, S.; Eeckhaut, A.V.; Smolders, I.; Michotte, Y. Critical evaluation of acetylcholine determination in rat brain microdialysates using ion-pair liquid chromatography with amperometric detection. Sensors 2008, 8, 5171–5185. [Google Scholar] [CrossRef]
- Schuvailo, S.V.; Dzyadevych, A.V.; Elskaya, A.V.; Gautier-Sauvigne, S.; Csoregi, E.; Cespuglio, R.; Soldatkin, A.P. Carbon fibre-based microbiosensors for in vivo measurements of acetylcholine and choline. Biosens. Bioelectron. 2005, 21, 87–94. [Google Scholar] [CrossRef]
- Carballo, R.; Orto, V.C.D.; Rezzano, I. Poly [Ni (II) protoporphyrin IX] modified electrode for amperometric detection of acetylcholine (Ach) and choline (Ch). Anal. Lett. 2007, 40, 1962–1971. [Google Scholar] [CrossRef]
- Jaffrezic-Renault, N.; Dzyadevych, S.V. Conductometric microbiosensors for environmental monitoring. Sensors 2008, 8, 2569–2588. [Google Scholar] [CrossRef]
- Periasamy, A.P.; Umasankar, Y.; Chen, S.M. Nanomaterials-acetylcholinesterase enzyme matrices for organophosphorus pesticides electrochemical sensors—A Review. Sensors 2009, 9, 4034–4055. [Google Scholar] [CrossRef]
- Moreira, F.T.C.; Guerreiro, J.R.L.; Azevedo, V.L.; Kamel, A.H.; Sales, M.G.F. New biomimetic sensors for the determination of tetracycline in biological samples: Batch and flow mode operations. Anal. Methods 2010, 2, 2039–2045. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Mahmoud, W.H.; Mohamed, A.H.K.; Kelany, A.E. Mercury(II) Ion-Selective Polymeric Membrane Sensors for Analysis of Mercury in Hazardous Wastes. Anal. Sci. 2006, 22, 877–881. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Elnemma, E.M.; Mohamed, A.H.K. Novel Biomedical Sensors for Flow Injection Potentiometric Determination of Creatinine in Human Serum. Electroanalysis 2005, 17, 2246–2253. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Marzouk, S.A.M.; Mohamed, A.H.K.; Badawy, N.M. Novel dicyanoargentate polymeric membrane sensors for selective determination of cyanide ions. Electroanalysis 2004, 16, 298–303. [Google Scholar] [CrossRef]
- Kamel, A.H.; Galal, H.R.; Awaad, N.S. Cost-effective and handmade paper-based potentiometric sensing platform for piperidine determination. Anal. Methods 2018, 10, 5406–5415. [Google Scholar] [CrossRef]
- Kamel, A.H.; Jiang, X.; Li, P.; Liang, R. A paper-based potentiometric sensing platform based on molecularly imprinted nanobeads for determination of bisphenol A. Anal. Methods 2018, 10, 3890–3895. [Google Scholar] [CrossRef]
- Kamel, A.H.; Hassan, A.M.E. Solid Contact Potentiometric Sensors Based on Host-Tailored Molecularly Imprinted Polymers for Creatine Assessment. Int. J. Electrochem. Sci. 2016, 11, 8938–8949. [Google Scholar] [CrossRef]
- Jaramillo, A.; Lopez, S.; Justice, J.B. Acetycholine and choline ion-selective microelectrodes. Anal. Chem. Acta 1983, 146, 149–159. [Google Scholar] [CrossRef]
- Kima, H.; Oha, J.; Jeona, W.S.; Selvapalam, N.; Hwang, I.; Koa, Y.H.; Kim, K. A new cucurbit[6]uril-based ion-selective electrode for acetylcholine with high selectivity over choline and related quaternary ammonium ions. Supramol. Chem. 2012, 24, 487–491. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.H.; Wu, Z.Y.; Wang, H.; Shen, G.L.; Yu, R.Q. A potentiometric acetylcholinesterase biosensor based on plasma-polymerized film. Sens. Actuators B 2005, 104, 186–200. [Google Scholar] [CrossRef]
- Poels, I.; Nagels, L. Potentiometric detection of amines in ion chromatography using macrocycle-based liquid membrane electrodes. Anal. Chim. Acta 2001, 440, 89–98. [Google Scholar] [CrossRef]
- Khaled, E.; Hassan, H.N.A.; Mohamed, G.G.; Ragab, F.A.; Seleim, A.A. β-Cyclodextrin-based potentiometric sensors for flow-injection determination of acetylcholines. Int. J. Electrochem. Sci. 2010, 5, 448–458. [Google Scholar]
- Kamel, A.H.; Al Hamid, F.A.; Soror, T.Y.; Galal, H.R.; Gendy, F.A. Solid contact biosensor based on man-tailored polymers for acetylcholine detection: Application to acetycholinesterase assay. Eur. Chem. Bull. 2016, 5, 266–273. [Google Scholar]
- Sacramento, A.S.; Moreira, F.T.C.; Guerreiro, J.L.; Tavares, A.P.; Sales, M.G.F. Novel biomimetic composite material for potentiometric screening of acetylcholine, a neurotrans- mitter in Alzheimer’s disease. Mater. Sci. Eng. C 2017, 79, 541–549. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Bakker, E. Determination of improved selectivity coefficients of polymer membrane ion-selective electrodes by conditioning with a discriminated ion. J. Electrochem. Soc. 1996, 143, L83–L85. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Veder, J.; De Marco, R.; Clarke, G.; Chester, R.; Nelson, A.; Prince, K.; Pretsch, E.; Bakker, E. Elimination of undesirable water layers in solid-contact polymeric ionselective electrodes. Anal. Chem. 2008, 80, 6731–6740. [Google Scholar] [CrossRef]
- Brunton, L.L. The Pharmacological Basis of Therapeutics, 11th ed.; McGraw Hill: New York, NY, USA, 2006. [Google Scholar]
- Royani, I.; Widayani, I.; Abdullah, M.; Khairurrijal, K. An atrazine molecularly imprinted polymer synthesized using a cooling-heating method with repeated washing: Its physico-chemical characteristics and enhanced cavities. Int. J. Electrochem. Sci. 2014, 9, 5651–5662. [Google Scholar]
Sample Availability: Samples of the compounds are not available. |






| Ionophore | Slope, (mV Decade−1) | Linear Range, (M) | Detection Limit, (M) | pH Range | Interference | Ref. |
|---|---|---|---|---|---|---|
| Acetylcholine dipicrylaminate | 54.4 | 5.0 × 10−5–1.0 × 10−2 | 3.0 × 10−5 | NR | Choline (−1.35); butyrylcholine (−1.02); dopamine (−2.21); tyrosine (−2.39); aminobutyric acid (−2.82); carbachol (−1.43); amphetamine (−1.06); K+ (−2.65); NH4+ (−3.39) | [30] |
| Cucurbit(6)uril derivative | 49.1 | 1.0 × 10−6–1.0 × 10−3 | 9.70 × 10−7 | 7.2 | choline (−2.51); NH4+ (−1.96); NMe4+ (−1.93); NEt4+ (−1.93); K+ (−1.57); Na+ (−1.83); dopamine (−1.51); ascorbic acid (−2.45) | [31] |
| Dioctyloctadecylamine N,N-didecyl-aminomethyl -benzene | 41.4 52.9 | 3.0 × 10−6–4.5 × 10−5 1.0 × 10−5–8.0 × 10−3 | 2.0 × 10−6–5.0 × 10−6 5.0 × 10−6 | 8.0 8.0 | NR NR | [32] |
| Tetrakis(p-chloro-phenyl) borate | NR | NR | 1.0 × 10−5 | 6 | NR | [33] |
| Dibenzo-18-crown-6 | NR | NR | 1.0 × 10−5 | 6 | NR | |
| Calix[6]arene hexaester | NR | NR | 1.7 × 10−5 | 6 | NR | |
| β-Cyclodextrin (β-CD) derivative | 55.6 | 1.00 × 10−5–1.0 × 10−2 | 2.70 × 10−6 | 3.0–10 | Choline (−2.50); NH4+ (−3.80); citrate (−2.53); Li+ (−3.76); K+ (−3.89); caffeine (−2.30) | [34] |
| Molecularly imprinted polymer (MIP) based on methacrylic acid monomer (MAA) and tetraphenylborate (TPB) additive | 55.2 | 1.00 × 10−5–1.0 × 10−2 | 4.5 × 10−6 | 3.0–9.0 | Glutamine (−1.52); codeine (−1.37); ephedrine (−1.45); morphine (−1.50); caffeine (−1.5); quinine (−1.57); histidine (−1.60); choline (−1.62); cysteine (−1.70); K+ (−2.51); Ca2+ (−2.54); Mg2+ (−2.82); Ba2+ (−2.93) | [35] |
| Multiwall carbon nanotubes (MWCNTs) and aniline (ANI) in bulk imprinting. | 75.9 | 3.45 × 10−5–1.0 × 10−2 | 3.13 × 10−5 | 4.0 | Creatinine (−0.04), creatine (+0.82), cysteine (+0.08), glutamine (−0.35), urea (−0.23) | [36] |
| ACh/TPB/PEDOT/PSS-ISE | 56.4 ± 0.6 | 1.00 × 10−6–10 × 10−3 | 2.0 × 10−7 | 3–10 | Choline (−3.3), urea (−4.2), hexamine (−4.3), ethylene diamine (−6.2), dimethylamine (−3.2), hydroxylamine (−3.1), methylamine (−3.3), histidine (−4.5), alanine (−4.4), ephedrine (−3.3), codeine (−2.7), morphine (−2.9), K+ (−5.3); Ca2+ (−6.8); Mg2+ (−7.1); Na+(−5.3) | This work |
| β-CD/PEDOT/PSS-ISE | 55.3 ± 1.1 | 2.00 × 10−6–1.00 × 10−3 | 3.2 × 10−7 | 4.5–10 | Choline (−3.5), urea (−4.5), hexamine (−4.1), ethylene diamine (−6.1), dimethylamine (−4.9), hydroxylamine (−4.8), methylamine (−4.5), histidine (−4.1), alanine (−4.3), ephedrine (−1.2), codeine (−1.0), morphine (−1.1), K+ (−5.1); Ca2+ (−7.1); Mg2+ (−7.3); Na+(−6.2). |
| Parameter | ACh/TPB/PEDOT/PSS-ISE | β-CD/PEDOT/PSS-ISE |
|---|---|---|
| Slope, (mV decade−1) * | 56.4 ± 0.6 | 55.3 ± 1.1 |
| Correlation coefficient, (R2) | 0.999 | 0.998 |
| Linearity range, M * | 1.0 × 10−6–1 × 10−3 | 2.0 × 10−6–1.0 × 10−3 |
| Detection limit, M * | 2.0 × 10−7 | 3.2 × 10−7 |
| Working range, (pH) | 3–10 | 4.5–10 |
| Response time, (s) | <10 | <10 |
| Accuracy, (%) | 99.2 | 99.3 |
| Precision, (%) | 0.6 | 0.7 |
| Interferents | log KpotACh,j | |
|---|---|---|
| ACh/TPB/PEDOT/PSS-ISE | β-CD/PEDOT/PSS-ISE | |
| Choline | −3.3 ± 0.4 | −3.5 ± 0.3 |
| Na+ | −5.3 ± 0.7 | −6.2 ± 0.4 |
| K+ | −5.3 ± 0.3 | −5.1 ± 0.1 |
| Ca2+ | −6.8 ± 0.4 | −7.1 ± 0.4 |
| Mg2+ | −7.1 ± 0.1 | −7.3 ± 0.6 |
| Urea | −4.2 ± 0.3 | −4.5 ± 0.7 |
| Hexamine | −4.3 ± 0.2 | −4.1 ± 0.6 |
| Ethylene diamine | −6.2 ± 0.2 | −6.1 ± 0.4 |
| Dimethylamine | −3.2 ± 0.2 | −4.9 ± 0.6 |
| Hydroxylamine | −3.1 ± 0.3 | −4.8 ± 0.2 |
| Methylamine | −3.3 ± 0.4 | −4.5 ± 0.3 |
| Histidine | −4.5 ± 0.3 | −4.1 ± 0.4 |
| Alanine | −4.4 ± 0.4 | −4.3 ± 0.2 |
| Ephedrine | −3.3 ± 0.4 | −1.2 ± 0.3 |
| Codeine | −2.7 ± 0.3 | −1.0 ± 0.2 |
| Morphine | −2.9 ± 0.6 | −1.1 ± 0.1 |
| Parameter | ACh/TPB/PEDOT/PSS-ISE | β-CD/PEDOT/PSS-ISE |
|---|---|---|
| Slope (mV/decade) * | 60.1 ± 1.1 | 52.7 ± 0.8 |
| Correlation coefficient | 0.995 | 0.996 |
| Detection limit, (M *) | 2.5 × 10−6 | 3.9 × 10−6 |
| Linear range, (M *) | 1.0 × 10−3–1.0 × 10−5 | 1.0 × 10−3–2.0 × 10−5 |
| Optimized flow rate, (mL/min) | 4.0 | 4.0 |
| Life span, (week) | 8 | 8 |
| Sample throughputs, (h) | 24–25 | 29–30 |
| Sample | Gender | Age | ACh Amount, µM * | |
|---|---|---|---|---|
| Potentiometry | Spectrophotometry | |||
| 1 | Male | 55–65 | 0.60 ± 0.03 | 0.52 ± 0.03 |
| 2 | Male | 35–50 | 0.48 ± 0.02 | 0.55 ± 0.04 |
| 3 | Male | 20–30 | 0.71 ± 0.01 | 0.57 ± 0.01 |
| 4 | Female | 40–55 | 0.46 ± 0.02 | 0.41 ± 0.02 |
| 5 | Female | 20–35 | 0.53 ± 0.04 | 0.46 ± 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashmawy, N.H.; Almehizia, A.A.; Youssef, T.A.; Amr, A.E.-G.E.; Al-Omar, M.A.; Kamel, A.H. Novel Carbon/PEDOT/PSS-Based Screen-Printed Biosensors for Acetylcholine Neurotransmitter and Acetylcholinesterase Detection in Human Serum. Molecules 2019, 24, 1539. https://doi.org/10.3390/molecules24081539
Ashmawy NH, Almehizia AA, Youssef TA, Amr AE-GE, Al-Omar MA, Kamel AH. Novel Carbon/PEDOT/PSS-Based Screen-Printed Biosensors for Acetylcholine Neurotransmitter and Acetylcholinesterase Detection in Human Serum. Molecules. 2019; 24(8):1539. https://doi.org/10.3390/molecules24081539
Chicago/Turabian StyleAshmawy, Nashwa H., Abdulrahman A. Almehizia, Teraze A. Youssef, Abd El-Galil E. Amr, Mohamed A. Al-Omar, and Ayman H. Kamel. 2019. "Novel Carbon/PEDOT/PSS-Based Screen-Printed Biosensors for Acetylcholine Neurotransmitter and Acetylcholinesterase Detection in Human Serum" Molecules 24, no. 8: 1539. https://doi.org/10.3390/molecules24081539









