Synthesis and Antiproliferative Activity of Novel Heterocyclic Glycyrrhetinic Acid Derivatives
Abstract
:1. Introduction
2. Results and Discussion
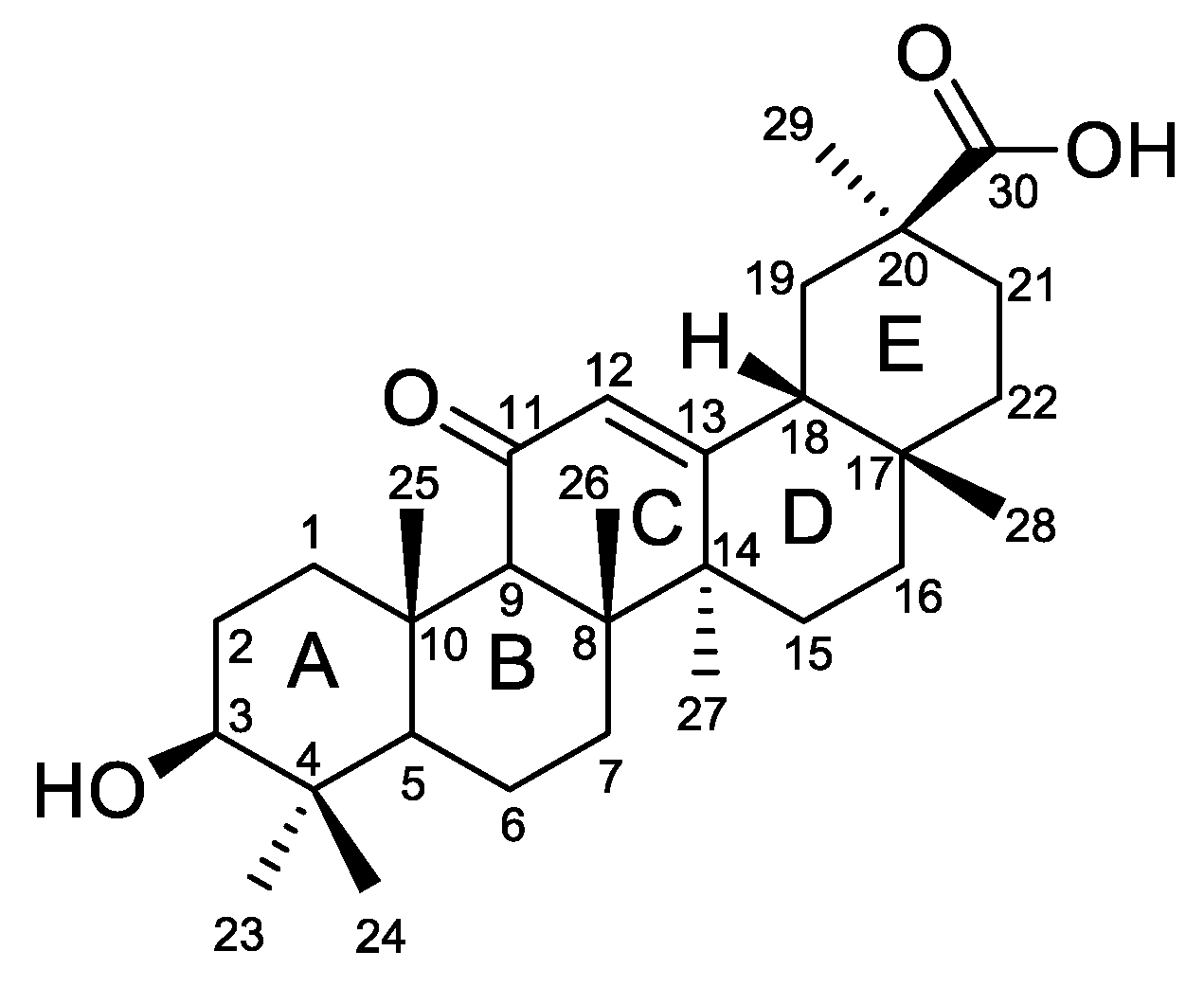
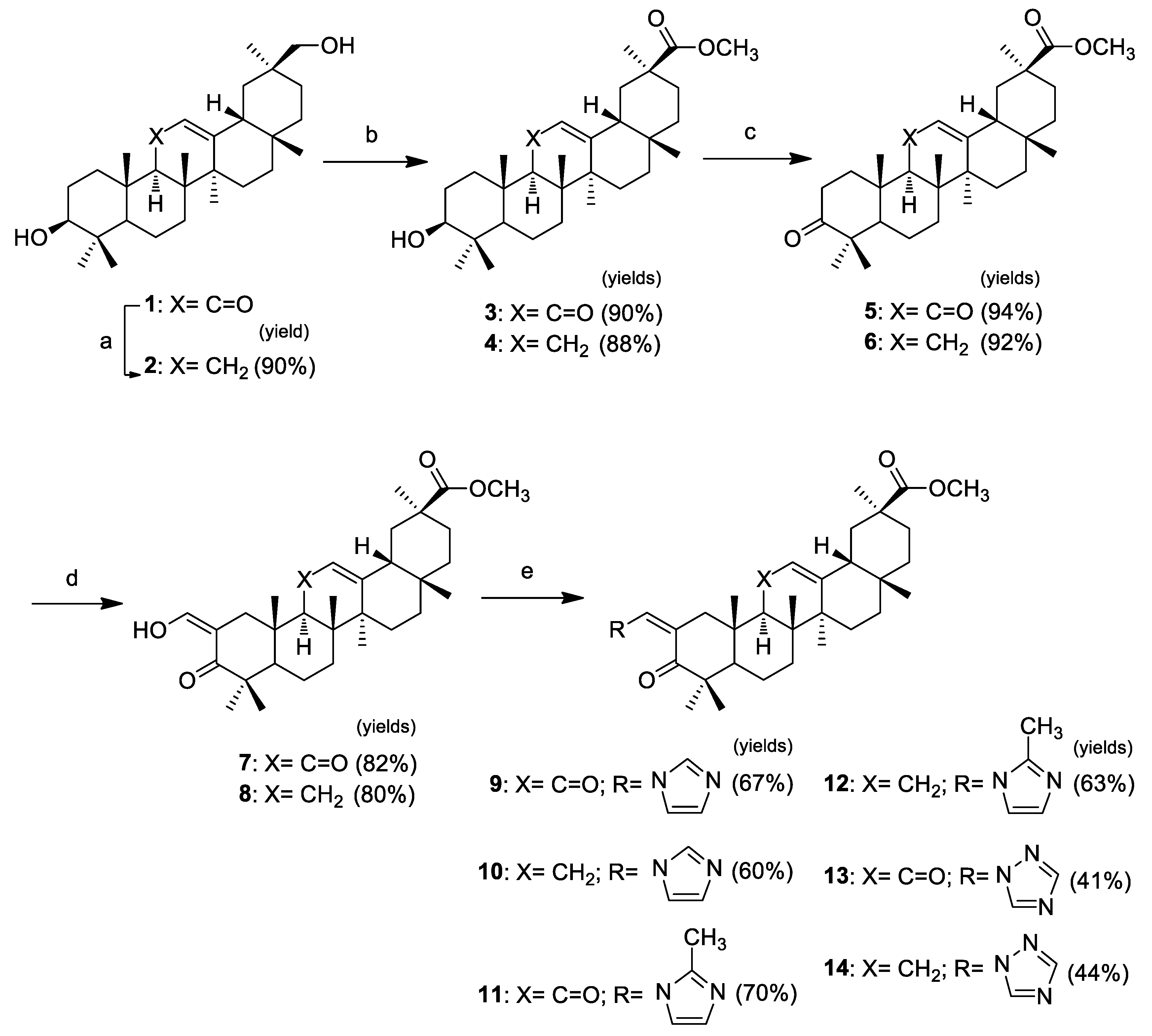
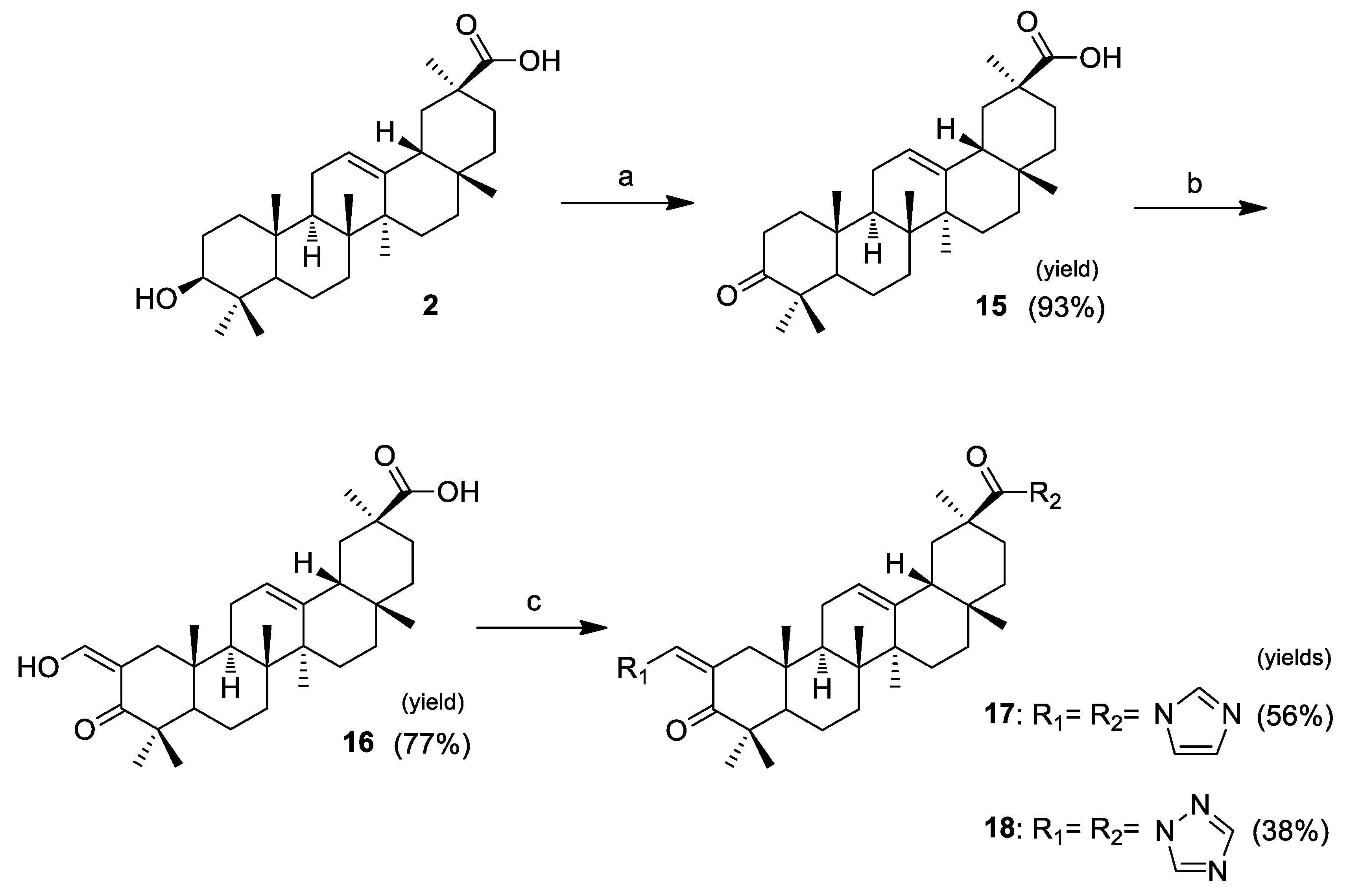
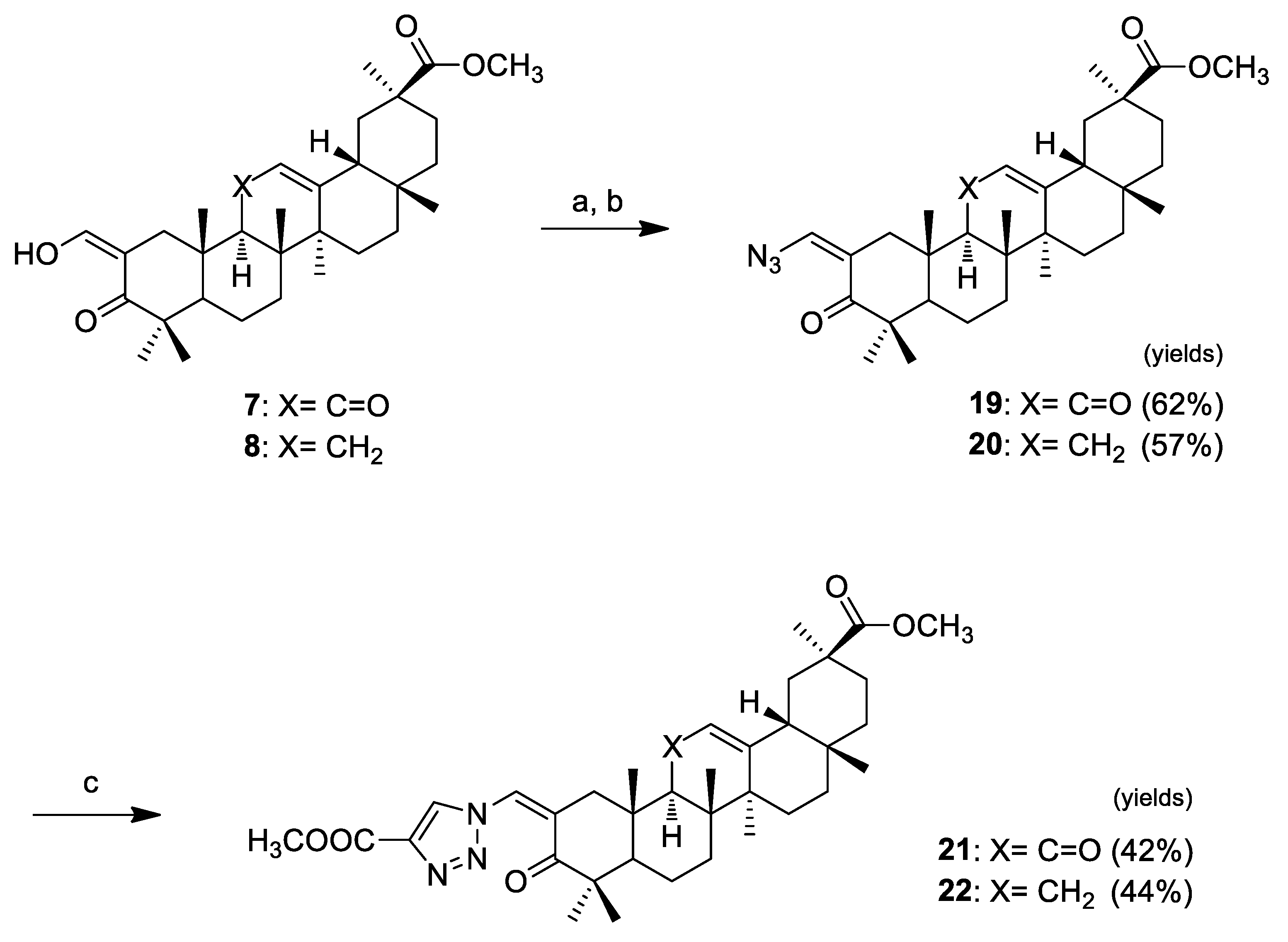
2.1. Chemistry
2.2. Biological Activity
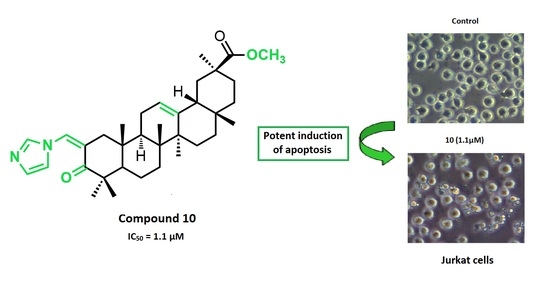
2.2.1. Antiproliferative Activity
2.2.2. Cell Viability over Time
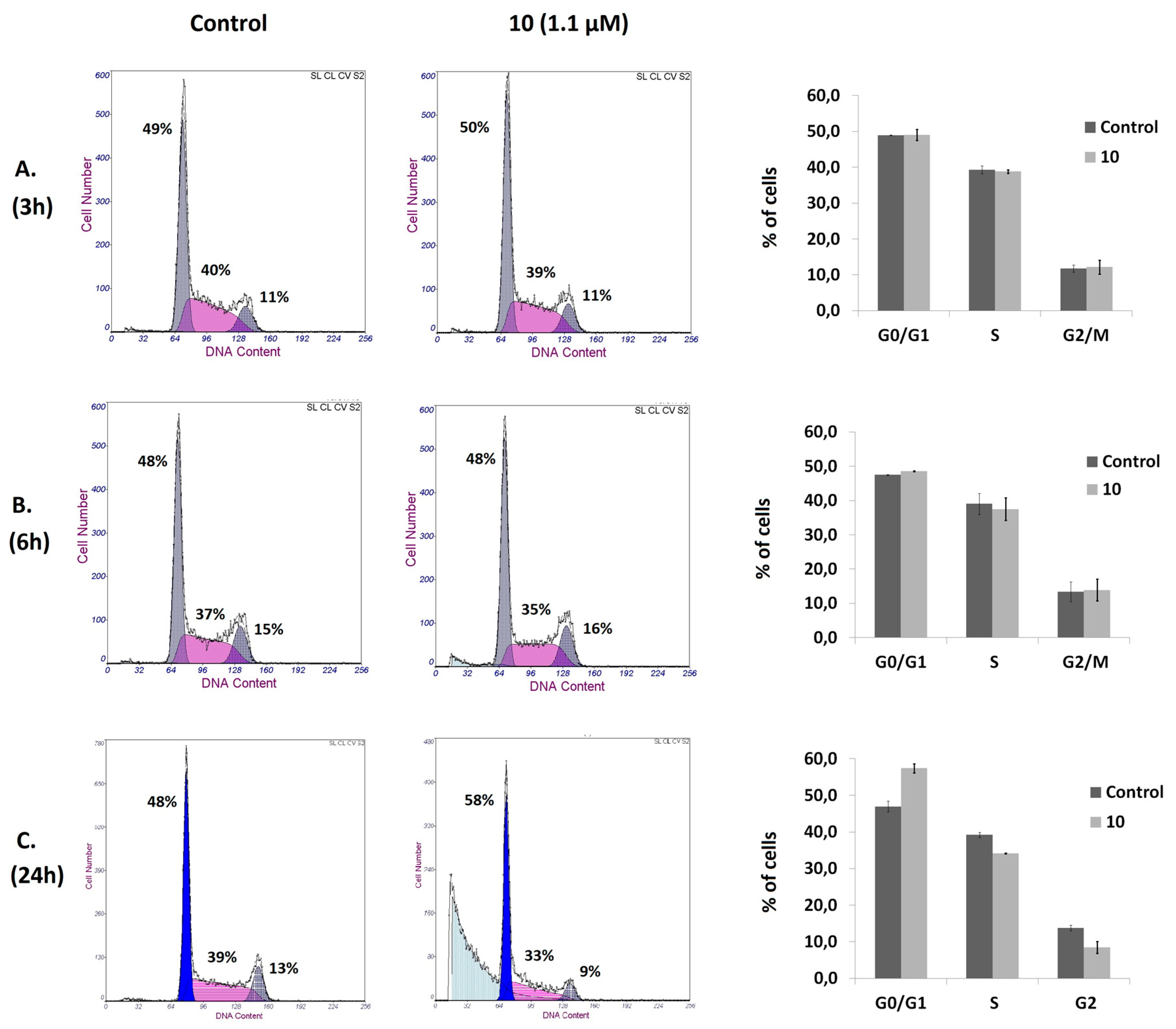
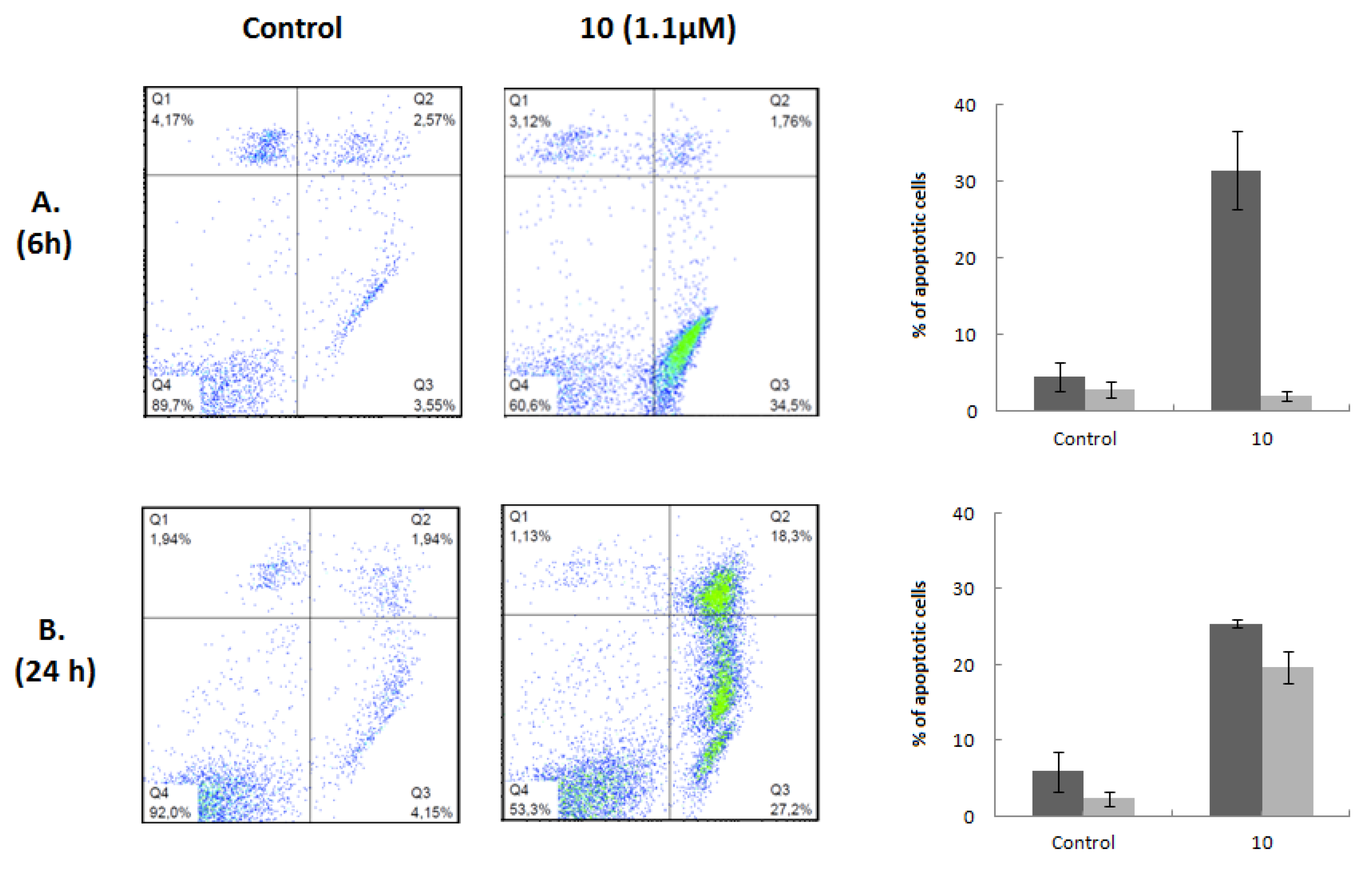
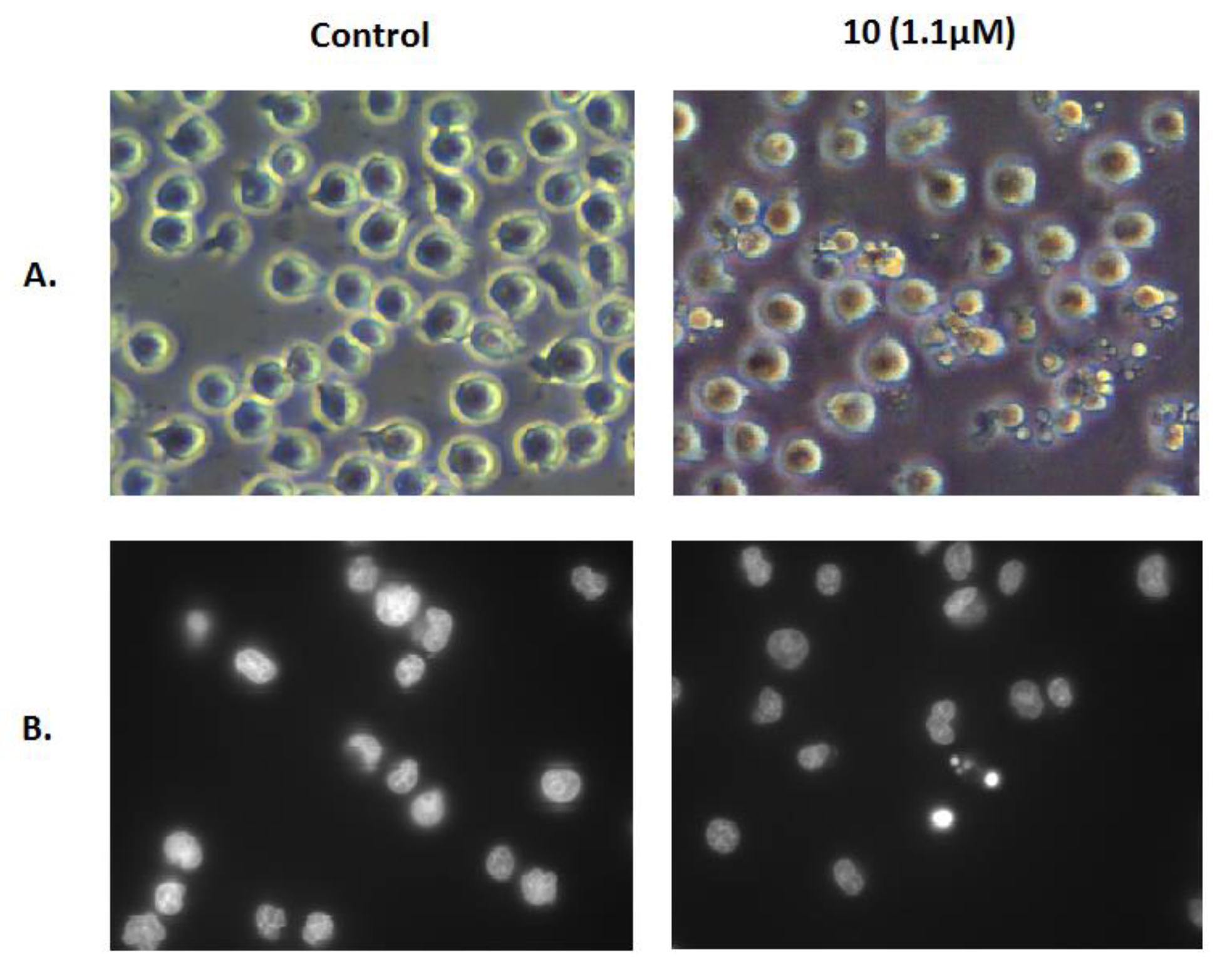
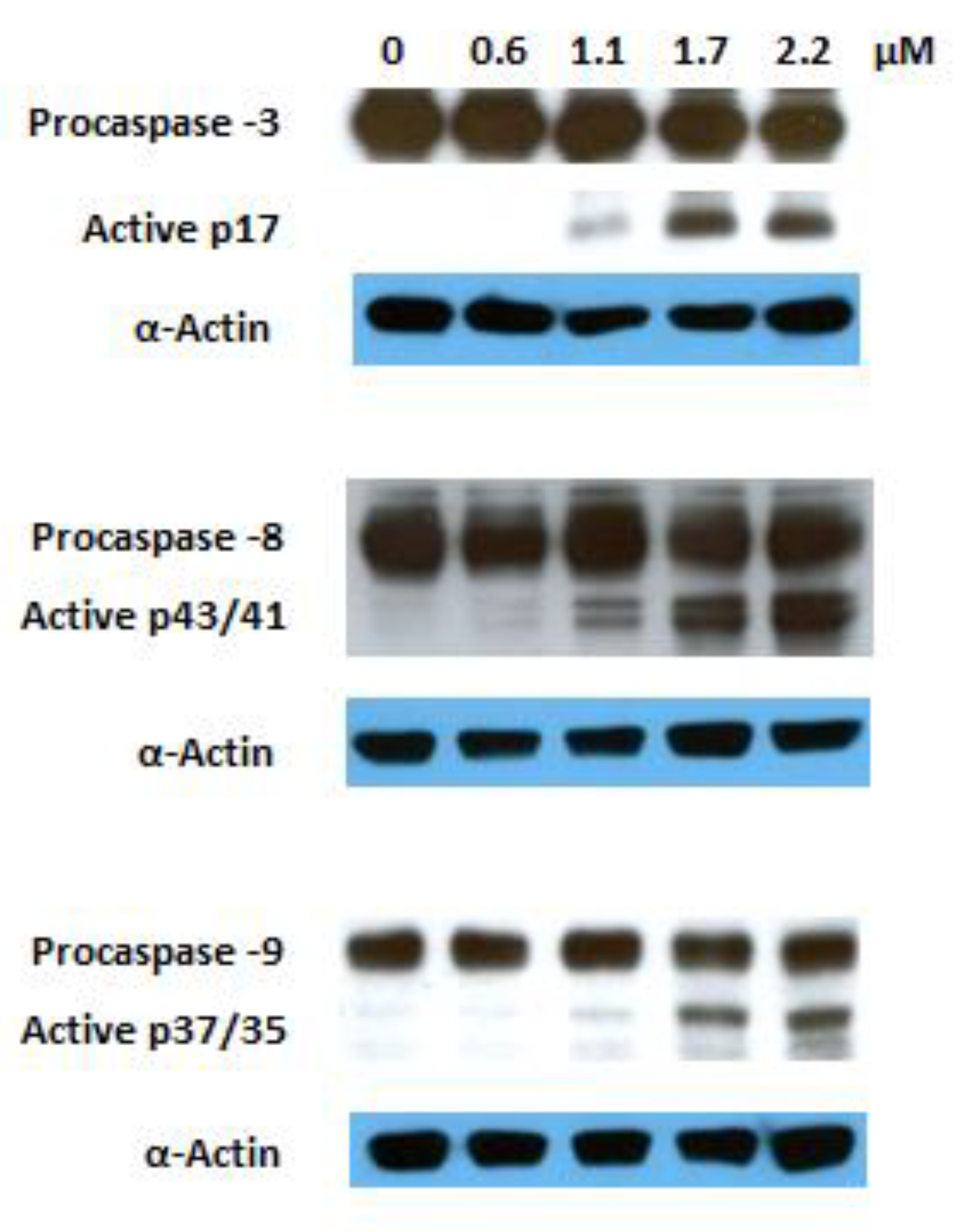
2.2.3. Analysis of Cell Cycle Distribution and Apoptosis
3. Materials and Methods
3.1. Chemistry
3.2. Biology
3.2.1. Cell Culture
3.2.2. Antiproliferative Activity Assays
3.2.3. Cell Viability over Time Assays
3.2.4. Cell Cycle Analysis
3.2.5. Annexin V-FITC/PI Flow Cytometry Assay
3.2.6. Observation of Morphological Changes
3.2.7. Western Blot Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.R.; Moreira, V.M.; Goncalves, B.M.F.; Leal, A.S.; Jing, Y.K. Ursane-type pentacyclic triterpenoids as useful platforms to discover anticancer drugs. Nat. Prod. Rep. 2012, 29, 1463–1479. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.R.; Leal, A.S.; Alho, D.P.S.; Goncalves, B.M.F.; Valdeira, A.S.; Mendes, V.I.S.; Jing, Y.K. Highlights of pentacyclic triterpenoids in the cancer settings. In Studies in Natural Products Chemistry; AttaUrRahman, F.R.S., Ed.; Elsevier Science Bv: Amsterdam, The Netherland, 2014; Volume 41, pp. 33–73. [Google Scholar]
- Chudzik, M.; Korzonek-Szlacheta, I.; Krol, W. Triterpenes as potentially cytotoxic compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Goncalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Heller, L.; Schwarz, S.; Per, V.; Kowitsch, A.; Siewert, B.; Csuk, R. Incorporation of a michael acceptor enhances the antitumor activity of triterpenoic acids. Eur. J. Med. Chem. 2015, 101, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, B.M.F.; Salvador, J.A.R.; Marin, S.; Cascante, M. Synthesis and anticancer activity of novel fluorinated asiatic acid derivatives. Eur. J. Med. Chem. 2016, 114, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.I.S.; Bartholomeusz, G.A.; Ayres, M.; Gandhi, V.; Salvador, J.A.R. Synthesis and cytotoxic activity of novel a-ring cleaved ursolic acid derivatives in human non-small cell lung cancer cells. Eur. J. Med. Chem. 2016, 123, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Sommerwerk, S.; Heller, L.; Kuhfs, J.; Csuk, R. Urea derivates of ursolic, oleanolic and maslinic acid induce apoptosis and are selective cytotoxic for several human tumor cell lines. Eur. J. Med. Chem. 2016, 119, 1–16. [Google Scholar] [CrossRef]
- Sommerwerk, S.; Heller, L.; Kerzig, C.; Kramell, A.E.; Csuk, R. Rhodamine b conjugates of triterpenoic acids are cytotoxic mitocans even at nanomolar concentrations. Eur. J. Med. Chem. 2017, 127, 1–9. [Google Scholar] [CrossRef]
- Spivak, A.; Khalitova, R.; Nedopekina, D.; Dzhemileva, L.; Yunusbaeva, M.; Odinokov, V.; D’Yakonov, V.; Dzhemilev, U. Synthesis and evaluation of anticancer activities of novel c-28 guanidine-functionalized triterpene acid derivatives. Molecules 2018, 23, 22. [Google Scholar] [CrossRef]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of glycyrrhiza sp and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.H.; Sethi, G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, P.H.; Zhao, W.X.; Su, W.X.; Zhang, Z.Q.; Zhang, L.; Liu, J.M.; Ren, G.L.; Yin, Z.Y.; Wang, X.M. 18 beta-glycyrrhetinic acid inhibits hepatocellular carcinoma development by reversing hepatic stellate cell-mediated immunosuppression in mice. Int. J. Cancer 2013, 132, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.K.; Khan, R.; Ali, N.; Khan, A.Q.; Rehman, M.U.; Tahir, M.; Lateef, A.; Nafees, S.; Mehdi, S.J.; Rashid, S.; et al. 18-glycyrrhetinic acid alleviates 2-acetylaminofluorene-induced hepatotoxicity in wistar rats: Role in hyperproliferation, inflammation and oxidative stress. Hum. Exp. Toxicol. 2015, 34, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Shen, Y.; Qiu, R.F.; Chen, Z.L.; Chen, Z.H.; Chen, W.B. 18 beta-glycyrrhetinic acid exhibits potent antitumor effects against colorectal cancer via inhibition of cell proliferation and migration. Int. J. Oncol. 2017, 51, 615–624. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, Y.J.; Lee, M.S.; Han, E.S.; Lee, S.J. 18 beta-glycyrrhetinic acid induces apoptotic cell death in siha cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity. Life Sci. 2008, 83, 481–489. [Google Scholar] [CrossRef]
- Sharma, G.; Kar, S.; Palit, S.; Das, P.K. 18 beta-glycyrrhetinic acid (concur) induces apoptosis through modulation of akt/foxo3a/bim pathway in human breast cancer mcf-7 cells. J. Cell. Physiol. 2012, 227, 1923–1931. [Google Scholar] [CrossRef]
- Wang, D.; Wong, H.K.; Feng, Y.B.; Zhang, Z.J. 18beta-glycyrrhetinic acid induces apoptosis in pituitary adenoma cells via ros/mapks-mediated pathway. J. Neuro-Oncol. 2014, 116, 221–230. [Google Scholar] [CrossRef]
- Hasan, S.K.; Siddiqi, A.; Nafees, S.; Ali, N.; Rashid, S.; Ali, R.; Shahid, A.; Sultana, S. Chemopreventive effect of 18 beta-glycyrrhetinic acid via modulation of inflammatory markers and induction of apoptosis in human hepatoma cell line (hepg2). Mol. Cell. Biochem. 2016, 416, 169–177. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, B.X.; Liang, Q.Y.; Zhang, Y.Q.; Cai, J.Y.; Li, G.F. The selective effect of glycyrrhizin and glycyrrhetinic acid on topoisomerase ii alpha and apoptosis in combination with etoposide on triple negative breast cancer mda-mb-231 cells. Eur. J. Pharmacol. 2017, 809, 87–97. [Google Scholar] [CrossRef]
- Baltina, L.A. Chemical modification of glycyrrhizic acid as a route to new bioactive compounds for medicine. Curr. Med. Chem. 2003, 10, 155–171. [Google Scholar] [CrossRef]
- Guo, W.B.; Yan, M.M.; Xu, B.; Chu, F.H.; Wang, W.; Zhang, C.Z.; Jia, X.H.; Han, Y.T.; Xiang, H.J.; Zhang, Y.Z.; et al. Design, synthesis, and biological evaluation of the novel glycyrrhetinic acid-cinnamoyl hybrids as anti-tumor agents. Chem. Cent. J. 2016, 10, 11. [Google Scholar] [CrossRef]
- Li, Y.; Feng, L.; Song, Z.F.; Li, H.B.; Huai, Q.Y. Synthesis and anticancer activities of glycyrrhetinic acid derivatives. Molecules 2016, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.L.; Bai, L.F.; Zhu, H.L.; Zhang, W.M.; Lv, P.C. Synthesis and biological evaluation of glycyrrhetic acid derivatives as potential vegfr2 inhibitors. ChemMedChem 2017, 12, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wu, G.R.; Zhang, X.Y.; Yan, M.M.; Zhao, R.; Xue, N.N.; Fang, K.; Wang, H.; Chen, M.; Guo, W.B.; et al. An overview of structurally modified glycyrrhetinic acid derivatives as antitumor agents. Molecules 2017, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Y.; Huai, X.D.; Zheng, Q.X.; Wang, W.; Li, H.J.; Huai, Q.Y. Design and preparation of derivatives of oleanolic and glycyrrhetinic acids with cytotoxic properties. Drug Des. Dev. Ther. 2018, 12, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.C.; Salvador, J.A.R.; Marin, S.; Cascante, M.; Moreira, J.N.; Dinis, T.C.P. Synthesis and structure-activity relationship study of novel cytotoxic carbamate and n-acylheterocyclic bearing derivatives of betulin and betulinic acid. Bioorg. Med. Chem. 2010, 18, 4385–4396. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.S.; Wang, R.; Salvador, J.A.R.; Jing, Y.K. Synthesis of novel ursolic acid heterocyclic derivatives with improved abilities of antiproliferation and induction of p53, p21(waf1) and noxa in pancreatic cancer cells. Bioorg. Med. Chem. 2012, 20, 5774–5786. [Google Scholar] [CrossRef]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click chemistry for drug development and diverse chemical-biology applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Musumeci, F.; Schenone, S.; Desogus, A.; Nieddu, E.; Deodato, D.; Botta, L. Click chemistry, a potent tool in medicinal sciences. Curr. Med. Chem. 2015, 22, 2022–2050. [Google Scholar] [CrossRef]
- Ma, N.; Wang, Y.; Zhao, B.X.; Ye, W.C.; Jiang, S. The application of click chemistry in the synthesis of agents with anticancer activity. Drug Des. Dev. Ther. 2015, 9, 1585–1599. [Google Scholar] [CrossRef]
- Meghani, N.M.; Amin, H.H.; Leel, B.J. Mechanistic applications of click chemistry for pharmaceutical drug discovery and drug delivery. Drug Discov. Today 2017, 22, 1604–1619. [Google Scholar] [CrossRef]
- Pertino, M.W.; Lopez, C.; Theoduloz, C.; Schmeda-Hirschmann, G. 1,2,3-triazole-substituted oleanolic acid derivatives: Synthesis and antiproliferative activity. Molecules 2013, 18, 7661–7674. [Google Scholar] [CrossRef] [PubMed]
- Dangroo, N.A.; Singh, J.; Rath, S.K.; Gupta, N.; Qayum, A.; Singh, S.; Sangwan, P.L. A convergent synthesis of novel alkyne-azide cycloaddition congeners of betulinic acid as potent cytotoxic agent. Steroids 2017, 123, 1–12. [Google Scholar] [CrossRef]
- Pokorny, J.; Borkova, L.; Urban, M. Click reactions in chemistry of triterpenes - advances towards development of potential therapeutics. Curr. Med. Chem. 2018, 25, 636–658. [Google Scholar] [CrossRef] [PubMed]
- Pertino, M.W.; Petrera, E.; Alche, L.E.; Schmeda-Hirschmann, G. Synthesis, antiviral and cytotoxic activity of novel terpenyl hybrid molecules prepared by click chemistry. Molecules 2018, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Wang, Y.T.; Gao, Y.; Li, L.; Guo, X.; Liu, D.; Jing, Y.K.; Zhao, L.X. Synthesis of methyl 2-cyano-3,12-dioxo-18 beta-olean-1,9(11)-dien-30-oate analogues to determine the active groups for inhibiting cell growth and inducing apoptosis in leukemia cells. Org. Biomol. Chem. 2014, 12, 6706–6716. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Csuk, R. Synthesis and antitumour activity of glycyrrhetinic acid derivatives. Bioorg. Med. Chem. 2010, 18, 7458–7474. [Google Scholar] [CrossRef]
- Rao, G.; Kondaiah, P.; Singh, S.K.; Ravanan, P.; Sporn, M.B. Chemical modifications of natural triterpenes-glycyrrhetinic and boswellic acids: Evaluation of their biological activity. Tetrahedron 2008, 64, 11541–11548. [Google Scholar] [CrossRef]
- Chu, F.H.; Xu, X.; Li, G.L.; Gu, S.; Xu, K.; Gong, Y.; Xu, B.; Wang, M.N.; Zhang, H.Z.; Zhang, Y.Z.; et al. Amino acid derivatives of ligustrazine-oleanolic acid as new cytotoxic agents. Molecules 2014, 19, 18215–18231. [Google Scholar] [CrossRef] [PubMed]
- Porchia, M.; Dolmella, A.; Gandin, V.; Marzano, C.; Pellei, M.; Peruzzo, V.; Refosco, F.; Santini, C.; Tisato, F. Neutral and charged phosphine/scorpionate copper(i) complexes: Effects of ligand assembly on their antiproliferative activity. Eur. J. Med. Chem. 2013, 59, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, B.M.F.; Salvador, J.A.R.; Marin, S.; Cascante, M. Synthesis and biological evaluation of novel asiatic acid derivatives with anticancer activity. RSC Advances 2016, 6, 3967–3985. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Halliday, G.C.; Berry, P.; Rousseau, R.F.; Middleton, S.A.; Nichols, G.L.; Del Bello, F.; Piergentili, A.; Newell, D.R.; et al. Structurally diverse mdm2-p53 antagonists act as modulators of mdr-1 function in neuroblastoma. Br. J. Cancer 2014, 111, 716–725. [Google Scholar] [CrossRef]
- Antunovic, M.; Kriznik, B.; Ulukaya, E.; Yilmaz, V.T.; Mihalic, K.C.; Madunic, J.; Marijanovic, I. Cytotoxic activity of novel palladium-based compounds on leukemia cell lines. Anti-Cancer Drugs 2015, 26, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Rajic, Z.; Zorc, B.; Raic-Malic, S.; Ester, K.; Kralj, M.; Pavelic, K.; Balzarini, J.; De Clercq, E.; Mintas, M. Hydantoin derivatives of l- and d-amino acids: Synthesis and evaluation of their antiviral and antitumoral activity. Molecules 2006, 11, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 14. [Google Scholar] [CrossRef]
- Hibasami, H.; Iwase, H.; Yoshioka, K.; Takahashi, H. Glycyrrhetic acid (a metabolic substance and aglycon of glycyrrhizin) induces apoptosis in human hepatoma, promyelotic leukemia and stomach cancer cells. Int. J. Mol. Med. 2006, 17, 215–219. [Google Scholar] [CrossRef]
- Satomi, Y.; Nishino, H.; Shibata, S. Glycyrrhetinic acid and related compounds induce g1 arrest and apoptosis in human hepatocellular carcinoma hepg2. Anticancer Res. 2005, 25, 4043–4047. [Google Scholar]
- Lee, C.S.; Yang, J.C.; Kim, Y.J.; Jang, E.R.; Kim, W.; Myung, S.C. 18 beta-glycyrrhetinic acid potentiates apoptotic effect of trichostatin a on human epithelial ovarian carcinoma cell lines. Eur. J. Pharmacol. 2010, 649, 354–361. [Google Scholar] [CrossRef]
- Yang, J.C.; Myung, S.C.; Kim, W.; Lee, C.S. 18 beta-glycyrrhetinic acid potentiates hsp90 inhibition-induced apoptosis in human epithelial ovarian carcinoma cells via activation of death receptor and mitochondrial pathway. Mol. Cell Biochem. 2012, 370, 209–219. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.A.; Attardi, L.D. P53 at a glance. J. Cell Sci. 2010, 123, 2527–2532. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Haas, M. Frequent mutations in the p53 tumor suppressor gene in human leukemia t-cell lines. Mol. Cell Biol. 1990, 10, 5502–5509. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all compounds are available from the authors. |

| Compound | Cell line (IC50, µM) 1 | |
|---|---|---|
| HT-29 | A549 | |
| 1 | 115.7 ± 1.6 | 110.5 ± 3.9 |
| 2 | 88.1 ± 1.4 | N.D. |
| 3 | 19.6 ± 0.6 | N.D. |
| 4 | 18.5 ± 0.9 | N.D. |
| 5 | 46.3 ± 2.3 | N.D. |
| 6 | 63.9 ± 1.1 | N.D. |
| 7 | 14.3 ± 0.3 | 18.5 ± 1.5 |
| 8 | 14.0 ± 0.2 | 17.0 ± 0.6 |
| 9 | 11.5 ± 0.5 | 11.1 ± 0.1 |
| 10 | 3.3 ± 0.2 | 2.8 ± 0.2 |
| 11 | 9.4 ± 0.7 | 10.3 ± 0.6 |
| 12 | 3.6 ± 0.1 | 3.1 ± 0.1 |
| 13 | 31.2 ± 1.5 | 24.7 ± 0.9 |
| 14 | 12.1 ± 0.2 | 12.3 ± 0.6 |
| 15 | 92.3 ± 1.5 | N.D. |
| 16 | 60.3 ± 2.0 | 54.4 ± 2.6 |
| 17 | 22.4 ± 0.5 | 23.1 ± 0.8 |
| 18 | 21.8 ± 1.6 | 24.5 ± 0.6 |
| 19 | 38.5 ± 0.6 | 48.5 ± 0.8 |
| 20 | 36.3 ± 1.4 | 44.3 ± 3.6 |
| 21 | 11.0 ± 0.8 | 10.6 ± 0.1 |
| 22 | 8.9 ± 0.5 | 7.9 ± 0.4 |
| Cisplatin | 6.1 [41] | 12.6 ± 0.8 [42] |
| Compound | Cell line (IC50, µM) 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| MIAPaca2 | HeLa | A375 | MCF7 | HepG2 | SH-SY5Y | Jurkat | BJ | |
| 1 | 101.6 ± 1.6 | 107.2 ± 2.5 | 112.2 ± 2.6 | 97.8 ± 3.9 | 125.1 ± 9.1 | 109.7 ± 2.5 | 105.8 ± 5.0 | 165.0 ± 7.1 |
| 9 | 14.2 ± 0.9 | 12.4 ± 1.2 | 10.4 ± 1.0 | 6.4 ± 0.3 | 14.3 ± 0.3 | 6.0 ± 0.2 | 3.2 ± 0.1 | N.D. |
| 10 | 3.3 ± 0.2 | 2.2 ± 0.1 | 2.0 ± 0.2 | 3.0 ± 0.2 | 3.1 ± 0.1 | 1.7 ± 0.15 | 1.1 ± 0.06 | 6.9 ± 0.07 |
| 11 | 10.8 ± 0.2 | 10.9 ± 1.0 | 7.1 ± 0.3 | 5.6 ± 0.3 | 13.5 ± 0.4 | 5.6 ± 0.2 | 2.4 ± 0.1 | N.D. |
| 12 | 3.3 ± 0.2 | 2.6 ± 0.2 | 2.3 ± 0.2 | 3.2 ± 0.3 | 3.5 ± 0.2 | 2.2 ± 0.2 | 1.3 ± 0.12 | N.D. |
| 13 | 28.7 ± 2.6 | 28.9 ± 1.7 | 26.5 ± 0.9 | N.D. | N.D. | N.D. | N.D. | N.D. |
| 14 | 13.4 ± 0.5 | 11.8 ± 0.7 | 10.3 ± 1.0 | N.D. | N.D. | N.D. | N.D. | N.D. |
| 17 | 17.8 ± 1.1 | 22.2 ± 0.8 | 17.9 ± 0.2 | N.D. | N.D. | N.D. | N.D. | N.D. |
| 18 | 15.2 ± 0.5 | 17.9 ± 0.6 | 13.4 ± 1.0 | N.D. | N.D. | N.D. | N.D. | N.D. |
| 21 | 12.0 ± 1.0 | 10.6 ± 0.1 | 7.2 ± 0.5 | 6.0 ± 0.3 | 11.8 ± 0.4 | 3.7 ± 0.1 | 1.7 ± 0.10 | N.D. |
| 22 | 6.9 ± 0.3 | 5.4 ± 0.3 | 4.9 ± 0.1 | 5.2 ± 0.2 | 9.0 ± 0.1 | 3.2 ± 0.1 | 1.5 ± 0.12 | N.D. |
| Cisplatin | 5.0 ± 1.0 [46] | 2.3 ± 0.3 [43] | 3.1 ± 1.0 [42] | 19.1 ± 4.5 [43] | 2.9 [41] | 0.7 ± 0.1 [44] | 1.9 [45] | 10.1 ± 2.0 [43] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alho, D.P.S.; Salvador, J.A.R.; Cascante, M.; Marin, S. Synthesis and Antiproliferative Activity of Novel Heterocyclic Glycyrrhetinic Acid Derivatives. Molecules 2019, 24, 766. https://doi.org/10.3390/molecules24040766
Alho DPS, Salvador JAR, Cascante M, Marin S. Synthesis and Antiproliferative Activity of Novel Heterocyclic Glycyrrhetinic Acid Derivatives. Molecules. 2019; 24(4):766. https://doi.org/10.3390/molecules24040766
Chicago/Turabian StyleAlho, Daniela P. S., Jorge A. R. Salvador, Marta Cascante, and Silvia Marin. 2019. "Synthesis and Antiproliferative Activity of Novel Heterocyclic Glycyrrhetinic Acid Derivatives" Molecules 24, no. 4: 766. https://doi.org/10.3390/molecules24040766